Hydrothermal synthesis of well-crystallized CuO hierarchical structures and their direct application in high performance lithium-ion battery electrodes without further calcination†
Hequan Wanga,
Tangwen Liangac,
Xiao Yuc,
Wenxia Zhaob,
Ruimei Xub,
Donghai Wangc and
Yong Liu*c
aSchool of Mechatronics Engineering, Shenyang Aerospace University, Shenyang 110136, China
bInstrumental Analysis & Research Center, Sun Yat-sen University, Guangzhou 510275, China
cInstitute for Solar Energy System, State Key Laboratory of Optoelectronic Materials and Technologies, Sun Yat-sen University, Guangzhou 510275, China. E-mail: liuyong7@mail.sysu.edu.cn
First published on 7th October 2016
Abstract
The search for a facile and energy-saving nanomaterial fabrication technology is of great significance in the area of energy conversion and storage. However, most approaches require high temperature heat treatment to transform nanomaterials from amorphous to crystalline phase or improve their crystallinity. This paper reports a hydrothermal approach for the synthesis of well-crystallized CuO hierarchical structures assembled by intercrossed nanosheets. Time dependent experiments suggest that CuO hierarchical structures followed a rapid nucleation and crystal growth mechanism. Therefore, well-crystallized CuO hierarchical structures can be directly applied as anode for lithium-ion batteries without further calcination. The results show that the uncalcined CuO hierarchical structures could deliver discharge capacities of 575 mA h g−1 at 1C over 100 cycles and 504 mA h g−1 at 2C over 100 cycles, respectively, which were much better than those of calcined ones. This excellent performance can be ascribed to a synergistic effect of their high crystallinity and hierarchical structure containing micro and nano features.
1 Introduction
Lithium-ion batteries (LIBs) are widely used in portable electronic devices such as mobile phones, toys, laptops, watches, and are expected to be applied in electric vehicles and renewable energy storage in the near future.1 However, graphite, used in commercial LIB's anode, has very low capacity (372 mA h g−1) and Li-platting issues, which cannot meet requirements of high power LIBs.2 Ever since Tarascon's group have discovered that transition metal oxides (MxOy, where M is Co, Ni, Cu or Fe, etc.) can also storage lithium via conversion reaction, considerable efforts have been devoted to investigate them as alternative anode materials for graphite because of their high specific capacity (500–1000 mA h g−1).3–8 In particular, cupric oxide (CuO) material has attracted much more attention in recent years because it is an abundant, low cost, and environmentally benign material with relatively high theoretical capacity of 670 mA h g−1.9 It is generally considered that nanoengineering active materials can increase the electrode–electrolyte contact area and shorten the lithium-ion transport path, which can enhance the capacity and rate performance of LIBs.10 Therefore, various CuO nanostructures such as nanoparticles,11,12 nanospheres,13 nanowires,14 nanorods,15 nanotubes,16,17 nanosheets,18 and nanofibers19 have been obtained by chemical and physical methods. However, nanostructure materials incline to aggregate during galvanostatic cycling because of their high surface energy, which decreases the electrode–electrolyte contact area and prevents the diffusion of electrolyte to reach the surface of individual particles. In addition, they may also lead to low packing density and volume energy density of LIBs. As a result, a better strategy to solve this problem is to purposely assemble nano-sized building blocks into hierarchical structures, in which the micro-sized assemblies can effectively avoid aggregation and improve packing density while nano-sized building blocks may also expose more surface area to electrolyte and shorten lithium-ion diffusion path.20,21It is significant to find a simple and energy-saving procedure that can directly synthesize well-crystallized CuO nanostructures at low temperature and apply them in LIBs without further calcination. However, the most commonly used methods such as sol–gel, anodic oxidation, electro-deposition, template and hydrothermal/solvothermal route usually require high temperature heat treatment for transforming amorphous to crystalline phase, removing template or further improving crystallinity. For example, previous studies demonstrate a two-step fabrication process including the first hydrothermal synthesis of alkylamine-stabilized Cu nanowires and subsequent thermal annealing in air to prepare interconnected CuO nanotube networks.17 It also has been reported that Cu2O nanostructures were firstly fabricated using hydrothermal or other methods, and then transformed into CuO nanostructures by calcination in the range of 300–600 °C.15,22,23 In addition, some groups also reported that CuO nanostructures were fabricated by calcining Cu precursor (such as Cu(OH)2) in the temperature range of 300–800 °C.11,13,14,16,19,24–30 Wang et al. have systematically studied the lithium storage performance of leaf-like CuO, oatmeal-like CuO, and hollow-spherical CuO before and after calcination, and three different morphological samples after calcination are all superior to those before calcination, which are ascribed to the elimination of Cu(OH)2 impurities and good crystallinity in samples after calcination.31
Here, we present direct synthesis of well-crystallized CuO hierarchical structures that can be used as anode materials in LIBs without further calcination. The optimized 3D hierarchical architectures are composed of inter-crossed 2D nanosheets, which not only possess good robustness to ensure cycling stability through 3D assembly but also provide large electrode–electrolyte contact area and shorten lithium-ion diffusion via exposed 2D nanosheets. This unique stable structure provides high reversible capacity and excellent cycling stability. When evaluated as anode in lithium-ion batteries, CuO hierarchical structures exhibit high discharge capacities of 575 mA h g−1 at 1C over 100 cycles and 504 mA h g−1 at 2C over 100 cycles respectively, which were much better than those of calcined ones in this studies.
2 Experimental section
2.1 Synthesis
In a typical synthesis, 0.3 g of copper nitrate (Cu(NO3)2·3H2O) and 1 g polyvinyl pyrrolidone (PVP, 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MW) were added into a polytetrafluoroethene liner cup of a Teflon-lined stainless steel autoclave (100 ml) containing 32 mL of 1.56% (w/w) ammonia aqueous solution under vigorous stirring at room temperature. The autoclave was then sealed and heated to 200 °C for 24 h. After cooling to room temperature, the as-prepared precipitate was collected via centrifugation and washed with deionized water and absolute ethanol several times, and then dried in a vacuum at 60 °C for 24 h (denoted as uncalcined CuO hierarchical structures). The above as-synthesized sample was annealed at 500 °C for 3 h, which was donated as calcined CuO hierarchical structures. As another comparison, CuO nanosheet aggregates were fabricated by the direct combustion of copper nitrate (Cu(NO3)2·3H2O) powder in air at 600 °C for 2 h (donated as calcined CuO nanosheet aggregates). To investigate the formed CuO hierarchical structures mechanism, the other hydrothermal reaction time, such as 5 min, 15 min, 12 h was also performed.
000 MW) were added into a polytetrafluoroethene liner cup of a Teflon-lined stainless steel autoclave (100 ml) containing 32 mL of 1.56% (w/w) ammonia aqueous solution under vigorous stirring at room temperature. The autoclave was then sealed and heated to 200 °C for 24 h. After cooling to room temperature, the as-prepared precipitate was collected via centrifugation and washed with deionized water and absolute ethanol several times, and then dried in a vacuum at 60 °C for 24 h (denoted as uncalcined CuO hierarchical structures). The above as-synthesized sample was annealed at 500 °C for 3 h, which was donated as calcined CuO hierarchical structures. As another comparison, CuO nanosheet aggregates were fabricated by the direct combustion of copper nitrate (Cu(NO3)2·3H2O) powder in air at 600 °C for 2 h (donated as calcined CuO nanosheet aggregates). To investigate the formed CuO hierarchical structures mechanism, the other hydrothermal reaction time, such as 5 min, 15 min, 12 h was also performed.
2.2 Structure and electrochemical characterizations
Characterization techniques such as field emission scanning electron microscopy (FESEM, Quanata 400F, FEI, Japan), X-ray diffraction (XRD, PanAnalytic X'Pert Pro, Phillips), transmission electron microscopy (TEM, FEI Tecnai G20 microscopy at 200 kV) were used to study morphological feature and crystal structure of samples. The pore characteristics and specific surface area were obtained by a nitrogen adsorption–desorption apparatus (Auto ChemII 2920) at 77 K. The electrochemical performances were studied using standard CR2032-type coin cells with pure lithium metal serving as both the counter and reference electrodes. The slurry were composed of active material (e.g., uncalcined CuO hierarchical structures, calcined CuO hierarchical structures and calcined CuO nanosheet aggregates), conductivity agent (carbon black, Super-P-Li), and binder (polyvinylidene fluoride, PVDF, Aldrich) in a weight ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in N-methyl-2-pyrrolidone (NMP), which was then spread on Cu foil to prepare the working electrode. The mass loading of above active material in the electrode was controlled at about 1.4–1.6 mg cm−2. The electrodes were separated by Celgard 3400 membrane and filled with the electrolyte solution containing 1.0 M LiPF6 in a 1
1 in N-methyl-2-pyrrolidone (NMP), which was then spread on Cu foil to prepare the working electrode. The mass loading of above active material in the electrode was controlled at about 1.4–1.6 mg cm−2. The electrodes were separated by Celgard 3400 membrane and filled with the electrolyte solution containing 1.0 M LiPF6 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) mixture of ethylene carbonate–diethyl carbonate (Technologies, USA). Cell was assembled in an argon-filled glovebox (Mikrouna) with moisture and oxygen concentrations controlled below 0.1 ppm. The cyclic voltammetry (CV) curves were obtained by a CHI760C electrochemical workstation at a scan rate of 0.1 mV s−1. Galvanostatic charge–discharge cycling performance was measured on a NEWARE battery testing system between 0.01 and 3 V vs. Li at current rates in the ranges from 0.1 to 10C (1C = 670 mA g−1).
1 (w/w) mixture of ethylene carbonate–diethyl carbonate (Technologies, USA). Cell was assembled in an argon-filled glovebox (Mikrouna) with moisture and oxygen concentrations controlled below 0.1 ppm. The cyclic voltammetry (CV) curves were obtained by a CHI760C electrochemical workstation at a scan rate of 0.1 mV s−1. Galvanostatic charge–discharge cycling performance was measured on a NEWARE battery testing system between 0.01 and 3 V vs. Li at current rates in the ranges from 0.1 to 10C (1C = 670 mA g−1).
3 Results and discussion
Fig. 1a–c shows typical FESEM images of the uncalcined CuO hierarchical structures at different magnifications, which were obtained by hydrothermal treatment at 200 °C for 24 h. One can see that large-scale, monodisperse, and uniform hierarchical structures with a size of about 3.45 μm were obtained. FESEM image with higher magnification (Fig. 1c) reveals that these uncalcined CuO hierarchical structures are composed of a large number of intercrossed nanosheets with thickness of ca. 50 nm. Fig. S1† show the SEM images of calcined CuO hierarchical structures obtained after annealing at 500 °C for 3 h. It can be clearly seen that the overall morphology of CuO hierarchical structures can be well preserved after annealing treatment. As another comparison, CuO nanosheet aggregates were fabricated by the direct combustion of copper nitrate powder at 600 °C for 2 h, which have average size of 9.3 μm and sheet thickness of 200 nm (Fig. S2†). It can be seen from the XRD pattern (Fig. 1d) that uncalcined CuO hierarchical structure, calcined CuO hierarchical structure and calcined CuO nanosheet aggregate samples can all be indexed to the CuO phase (JCPDS no. 48-1548). Surprisingly, uncalcined CuO hierarchical structures have almost the same strong peak intensities as calcined ones, indicating their good crystallinity.The crystal structure of uncalcined CuO hierarchical structures was further investigated by TEM, corresponding selected area electron diffraction (SAED) and HRTEM measurements, as revealed in Fig. 2a–c. The SAED pattern exhibits the sharp diffraction spots along the [200] zone axis, which suggests nanosheets assembled in uncalcined CuO hierarchical structures are well crystallized and demonstrate the single crystalline feature. Fig. 2c shows that nanosheet assembled in uncalcined CuO hierarchical is single crystal, which is consistent with SAED result.
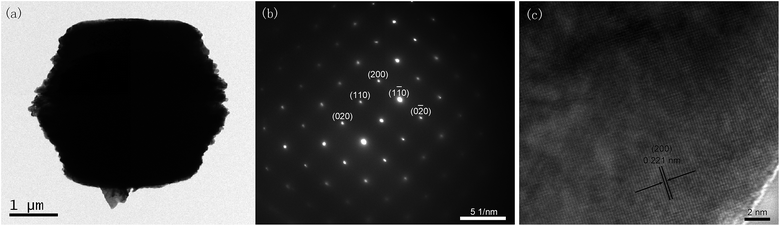 | ||
| Fig. 2 (a) TEM image, (b) SAED pattern and (c) HRTEM image of uncalcined CuO hierarchical structures. | ||
To better understand the formation process of CuO hierarchical structures, the as-synthesized products obtained with different hydrothermal reaction times were investigated by FESEM and XRD measurements. Fig. 3a–f shows the morphologies of CuO hierarchical structures with hydrothermal reaction time of 5 min, 15 min, and 12 h, respectively. Surprisingly, the samples prepared after 5 min hydrothermal reaction time have preliminarily formed hierarchical structures, which were composed of agglomerated nanosheets (Fig. 3a and b). It can be seen obviously that layered nanosheets began to appear more and more clearly, which were then intercrossed each other to assemble well-defined hierarchical structures while further increase reaction time to 15 min (Fig. 3c and d) and 12 h (Fig. 3e and f). Finally, when the reaction time reaches 24 h, CuO hierarchical structures are more uniform and elegant (see Fig. 1a–c) than those in Fig. 3.
 | ||
| Fig. 3 FESEM images of CuO hierarchical structures obtained at 200 °C with different hydrothermal reaction time (a) and (b) 5 min, (c) and (d) 15 min, and (e) and (f) 12 h. | ||
Therefore, we can reasonably infer that the nuclei and growth of CuO nanocrystals is fairly rapid. The further formation of CuO hierarchical structures may follow dissolution–recrystallization of primary agglomerated nanosheets.
Fig. 4 shows the XRD patterns of the above samples obtained after hydrothermal treatment for 5 min, 15 min, 12 h, and 24 h, respectively. All the XRD diffraction peaks of the samples in Fig. 4 can be indexed to CuO phase (JPCDS no. 48-1548), and the peak intensities of samples are almost same while increasing the reaction time. In addition, it should be noted that there is no intermediate precursors formed in the whole reaction process. Combined with SEM observations, it is reasonably assumed that the growth process involves the fast formation of CuO nanocrystals at the initial reaction stage and dissolution–recrystallization of primary agglomerated nanosheets in the subsequent process, resulting in the formation of well-crystallized CuO hierarchical structures.
 | ||
| Fig. 4 Time dependent XRD patterns of uncalcined CuO hierarchical structures obtained by hydrothermal treatment at 200 °C for 5 min, 15 min, 12 h, and 24 h, respectively. | ||
Fig. 5 shows the nitrogen adsorption–desorption isotherms (77 K) and their corresponding pore size distribution curves of uncalcined CuO hierarchical structures, calcined CuO hierarchical structures, and calcined CuO nanosheet aggregates, respectively. The Brunauer–Emmett–Teller (BET) specific surface area of uncalcined CuO hierarchical structures is 27.0 m2 g−1, which is much larger than those of calcined CuO hierarchical structures (4.4 m2 g−1) and calcined CuO nanosheet aggregates (3.3 m2 g−1). The Barrett–Joyner–Halenda (BJH) pore size distribution derived from the isotherm for these three samples all exhibits a narrow pore size distribution centered ranging from 1 to 10 nm (the inset in Fig. 5). However, the pole volume of the uncalcined CuO hierarchical structures is 0.06 cc g−1, which is larger than those of calcined CuO hierarchical structures (0.013 cc g−1), and calcined CuO nanosheet aggregates (0.005 cc g−1). Compared to uncalcined CuO hierarchical structures, the drastic decrease in specific surface areas and pore volume for calcined CuO hierarchical structures is probably due to crystalline growth and disappearance of mesoporous structures between layered nanosheets during calcination process.32
Then, the electrochemical properties of the uncalcined CuO hierarchical structures were evaluated. Fig. 6 shows the representative cyclic voltammograms (CVs) of the uncalcined CuO hierarchical structure electrode. During the first scan, the observed cathodic peak at 1.85 V, 1.04 V and 0.84 V were attributed to the multi-step electrochemical processes including the formation of an solid solution of CuII1−xCuIxO1−x/2 (0 < x < 0.4), a phase transition into Cu2O, and the final reduction to Cu and Li2O, respectively, which can be expressed as:28,31,33
| CuO + xLi+ + xe− → CuII1−xCuIxO1−x/2 + x/2Li2O (0 < x < 0.4) | (1) |
| CuII1−xCuIxO1−x/2 + (1−x)Li+ + (1−x)e− → ½Cu2O + (1−x)/2Li2O | (2) |
| ½Cu2O + Li+ + e− → Cu + ½Li2O | (3) |
In the reaction (1), the formation of the intermediate phase CuII1−xCuIxO1−x/2 (0 < x < 0.4) indicates the gradual reduction of Cu(II) in Cu(I) in the material. In addition, the oxygen, leaving the host structure and inducing oxygen ion vacancies creation, is trapped by lithium ion to form Li2O phase.34 In the first anodic scan process, two oxidation peaks appear at 2.44 and 2.75 V, which can be ascribed to the formation of Cu2O and CuO, as shown in eqn (4) and (5).20
| Cu + ½Li2O → ½Cu2O + Li + e− | (4) |
| ½Cu2O + ½Li2O → CuO + Li + e− | (5) |
Obviously, the CV curve in the first cycle is substantially different from those of the subsequent ones. It is obvious that cathodic peak potentials are shifted to 1.20 V and 0.86 V combined with large decreases of peak intensity and integral area after the second cycles, indicating the irreversible capacity loss occurs in this process. In addition, the overlapping of CV curves is observed from the second cycle onwards, which demonstrates the good cycle stability of CuO hierarchical structure electrode upon cycling.19
Fig. 7 shows the charge–discharge voltage profiles of the uncalcined CuO hierarchical structures, calcined CuO hierarchical structures and calcined CuO nanosheet aggregate electrodes at a current rate of 0.5C over the potential range of 0.01–3 V. It can be clearly seen from Fig. 7a that the obvious multiple plateaus are observed at 2.27–1.37 V, 1.37–0.92 V and 0.92–0.01 V in the discharge curves, whereas, a plateau centered at 2.49 V was noticed in the charge curves, which are also consistent with the peak observations in CV curves.13,20 In addition, an initial high discharge capacity of 947 mA h g−1 and subsequent charge capacity of 472 mA h g−1 were achieved for the uncalcined CuO hierarchical structure electrode, indicating an irreversible capacity loss of 49.8%. The irreversible capacity loss for the initial cycles is due to the conversion reaction process of metal oxides, and the irreversible formation of the solid electrolyte interphase (SEI), which were also commonly observed in other CuO electrodes.17,31 In the followed 5th, 10th, 30th and 50th cycles, the discharge and charge capacities were 565/555 mA h g−1, 600/581 mA h g−1, 565/553 mA h g−1 and 554/544 mA h g−1, respectively, corresponded to enhanced coulombic efficiency of 98.2%, 96.8%, 97.8% and 98.2%, respectively. There is no substantial change in the peak potentials and curve shape during successive cycles which demonstrates the good cycle stability of uncalcined CuO hierarchical structures. In contrast, the calcined CuO hierarchical structures and calcined CuO nanosheet aggregates presented much worse electrochemical lithium storage performance than the uncalcined CuO hierarchical structure electrode.
The cyclic performance of the uncalcined CuO hierarchical structures, calcined CuO hierarchical structures and calcined CuO nanosheet aggregates were further investigated at current rate of 1C and 2C, respectively, as shown in Fig. 8. The uncalcined CuO hierarchical structures could deliver discharge capacities of 575 mA h g−1 at 1C over 100 cycles and 504 mA h g−1 at 2C over 100 cycles, which were much better than those of calcined CuO hierarchical structures and calcined CuO nanosheet aggregates. These results are completely different from the previous studies. For instance, Wang et al. have systematically studied the lithium storage performance of leaf-like CuO, oatmeal-like CuO, and hollow-spherical CuO before and after calcination, and the samples after calcination were all superior to those before calcination.31 Qian's group have also reported that α-MoO3 after calcination demonstrated a better cycling stability than that before calcination.35 They all think that calcination can improve crystallinity and eliminate impurities, resulting in the enhanced lithium storage performance.31,35 In our studies, XRD and TEM measurements confirm that the uncalcined CuO hierarchical structures have the same crystallinity as the calcined ones, but the extra high-temperature heat treatment may cause thermal induced structural disorder, which leads to poor electrochemical performance.32,36 To reveal why the lithium storage of the uncalcined CuO hierarchical structure electrode is stable upon cycling, the morphologies of uncalcined CuO hierarchical structures were investigated after completion of 100 charge/discharge cycles at a current rate of 2C (Fig. S3†). Obviously, most of them were well preserved due to their robust structure, which ensured the excellent reversible capacity during cycles. The uncalcined CuO hierarchical structures demonstrate better reversible capacity and cycling performance than those of the previously reported CuO based materials treated with high temperature calcination,16,23,25–29,37,38 demonstrating a very promising candidate for next generation electrode of LIBs.
4 Conclusions
In summary, we demonstrated a simple route for fabricating well-crystallized CuO hierarchical structures by a hydrothermal process, which can be directly used as anode materials without further calcination. The CuO hierarchical architectures consists of intercrossed 2D nanosheets, which can possess robust structure, expose large electrode–electrolyte contact area and shorten lithium-ion diffusion path, thus resulting in excellent electrochemical properties, including a high reversible capacity, good cycling stability, and excellent rate capability. More importantly, a facile and energy-saving nanomaterial fabrication technology in this study is of great significance for its future application in LIBs.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 51272294), the Fundamental Research Funds for the Central Universities (16lgjc60) and Science and Technology Program of Guangzhou, China (No. 201508010011).Notes and references
- L. Croguennec and M. R. Palacin, J. Am. Chem. Soc., 2015, 137, 3140–3156 CrossRef CAS PubMed.
- F. Yao, D. T. Pham and Y. H. Lee, ChemSusChem, 2015, 8, 2284–2311 CrossRef CAS PubMed.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J.-M. Tarascon, Nature, 2000, 407, 496–499 CrossRef CAS PubMed.
- J. Cabana, L. Monconduit, D. Larcher and M. R. Palacin, Adv. Mater., 2010, 22, E170–E192 CrossRef CAS PubMed.
- S. H. Yu, S. H. Lee, D. J. Lee, Y. E. sung and T. Hyeon, Small, 2016, 12, 2146–2172 CrossRef CAS PubMed.
- J. Wang, Q. Zhang, X. Li, B. Zhang, L. Mai and K. Zhang, Nano Energy, 2015, 12, 437–446 CrossRef CAS.
- T. Li, X. Li, Z. Wang, H. Guo and Y. Li, J. Mater. Chem. A, 2015, 3, 11970 CAS.
- Q. Zhang, J. Wang, J. Dong, F. Ding, X. Li, B. Zhang, S. Yang and K. Zhang, Nano Energy, 2015, 13, 77–79 CrossRef CAS.
- M. V. Reddy, G. V. Subba Rao and B. V. R. Chowdari, Chem. Rev., 2013, 113, 5364–5457 CrossRef CAS PubMed.
- C. Jiang, E. Hosono and H. Zhou, Nano Today, 2006, 1, 28–33 CrossRef.
- L. Wang, K. Tang, M. Zhang, X. Zhang and J. Xu, Funct. Mater. Lett., 2014, 07, 1440008 CrossRef CAS.
- X. Zhang, D. Zhang, X. Ni, J. Song and H. Zheng, J. Nanopart. Res., 2008, 10, 839–844 CrossRef CAS.
- S. M. Abbas, S. T. Hussain, S. Ali, F. Abbas, N. Ahmad, N. Ali and Y. Khan, J. Alloys Compd., 2013, 574, 221–226 CrossRef CAS.
- R. Zhang, J. Liu, H. Guo and X. Tong, Mater. Lett., 2015, 139, 55–58 CrossRef CAS.
- H. Wang, Y. Zong, W. Zhao, L. Sun, L. Xin and Y. Liu, RSC Adv., 2015, 5, 49968–49972 RSC.
- A. Xiao, S. Zhou, C. Zuo, Y. Zhuan and X. Ding, Mater. Res. Bull., 2015, 70, 795–798 CrossRef CAS.
- J. Lee, S. Choi and S. Park, Chem.–Asian J., 2013, 8, 1377–1380 CrossRef CAS PubMed.
- Y. Liu, Y. Qiao, W. Zhang, P. Hu, C. Chen, Z. Li, L. Yuan, X. Hu and Y. Huang, J. Alloys Compd., 2014, 586, 208–215 CrossRef CAS.
- R. Sahay, P. S. Kumar, V. Aravindan, J. Sundaramurthy, W. C. Ling, S. G. Mhaisalkar, S. Ramakrishna and S. Madhavi, J. Phys. Chem. C, 2012, 116, 18087–18092 CAS.
- C. Wang, D. Higgins, F. Wang, D. Li, R. Liu, G. Xia, N. Li, Q. Li, H. Xu and G. Wu, Nano Energy, 2014, 9, 334–344 CrossRef CAS.
- Y. Zhou, Y. Liu, W. Zhao, R. Xu, D. Wang, B. Li, X. Zhou and H. Shen, Electrochim. Acta, 2016, 211, 1048–1055 CrossRef CAS.
- T. Wen, X.-L. Wu, S. Zhang, X. Wang and A.-W. Xu, Chem.–Asian J., 2015, 10, 595–601 CrossRef CAS PubMed.
- L. Feng, Z. Xuan, Y. Bai, H. Zhao, L. Li, Y. Chen, X. Yang, C. Su, J. Guo and X. Chen, J. Alloys Compd., 2014, 600, 162–167 CrossRef CAS.
- L. Wang, W. Cheng, H. Gong, C. Wang, D. Wang, K. Tang and Y. Qian, J. Mater. Chem., 2012, 22, 11297–11302 RSC.
- Z. Hu and H. Liu, Ceram. Int., 2015, 41, 8257–8260 CrossRef CAS.
- X. H. Huang, C. B. Wang, S. Y. Zhang and F. Zhou, Electrochim. Acta, 2011, 56, 6752–6756 CrossRef CAS.
- Y.-K. Kim, S. Cha, J.-H. Lee and S.-H. Hong, J. Nanosci. Nanotechnol., 2014, 14, 9143–9147 CrossRef CAS PubMed.
- R. Wu, X. Qian, F. Yu, H. Liu, K. Zhou, J. Wei and Y. Huang, J. Mater. Chem. A, 2013, 1, 11126–11129 CAS.
- X. Ma, S. Zeng, B. Zou, X. Liang, J. Liao and C. Chen, RSC Adv., 2015, 5, 57300–57308 RSC.
- A. Banerjee, U. Singh, V. Aravindan, M. Srinivasan and S. Ogale, Nano Energy, 2013, 2, 1158–1163 CrossRef CAS.
- C. Wang, Q. Li, F. Wang, G. Xia, R. Liu, D. Li, N. Li, J. S. Spendelow and G. Wu, ACS Appl. Mater. Interfaces, 2014, 6, 1243–1250 CAS.
- J. Yu, J. C. Yu, M. K.-P. Leung, W. Ho, B. Cheng, X. Zhao and J. Zhao, J. Catal., 2003, 217, 69–78 CAS.
- K. Chen and D. Xue, J. Phys. Chem. C, 2013, 117, 22576–22583 CAS.
- A. Debart, L. Dupont, P. Poizot, J.-B. Leriche and J. M. Tarascon, J. Electrochem. Soc., 2001, 148, A1266–A1274 CrossRef CAS.
- Y. Wang, Y. Zhu, Z. Xing and Y. Qian, Int. J. Electrochem. Sci., 2013, 8, 9851–9857 CAS.
- S. W. Xue, X. T. Zu, W. L. Zhou, H. X. Deng, X. Xiang, L. Zhang and H. Deng, J. Alloys Compd., 2008, 448, 21–26 CrossRef CAS.
- Y. Zhao, Y. Zhang, H. Zhao, X. Li, Y. Li, L. Wen, Z. Yan and Z. Huo, Nano Res., 2015, 8, 2763–2776 CrossRef CAS.
- X. Zhang, Y. Hu, D. Zhu, A. Xie and Y. Shen, Ceram. Int., 2016, 42, 1833–1839 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra20701d |
| This journal is © The Royal Society of Chemistry 2016 |





