Structural investigation of nickel polyphosphate coacervate glass–ceramics†
Douglas F. Franco*a,
Danilo Manzanib,
Hernane S. Barudc,
Selma G. Antoniob,
Luiz F. C. de Oliveirad,
Maurício A. P. Silvad,
Sidney J. L. Ribeirob and
Marcelo Nalinb
aLAVIE, Department of Chemistry, Federal University of São Carlos – UFSCar, CP 676, São Carlos, SP, Brazil. E-mail: fazafranco@yahoo.com.br
bInstitute of Chemistry, Universidade Estadual Paulista – Unesp, P. O. Box 355, Araraquara, SP 14801-970, Brazil
cLaboratório de Biopolímeros e Biomateriais (BioPolMat), Centro Universitário de Araraquara (UNIARA), Araraquara, SP, Brazil
dNúcleo de Espectroscopia e Estrutura Molecular – NEEM, Departamento de Química, Instituto de Ciências Exatas, Universidade Federal de Juiz de Fora – UFJF, 36036-900, Juiz de Fora, MG, Brazil
First published on 7th September 2016
Abstract
Nickel polyphosphate coacervates have been prepared through the coacervation process of sodium polyphosphate and Ni2+ chloride solutions by the addition of different solvents with low molecular weight. The great potential of nickel polyphosphate coacervates as a material precursor was demonstrated by preparation of nickel polyphosphate glass–ceramics. Structural and spectroscopic properties were analyzed by thermal analysis, X-ray powder diffraction, Raman spectroscopic and UV-VIS techniques. Raman spectra for the nickel polyphosphate coacervates and nickel polyphosphate coacervates glass–ceramics samples shows that the addition of solvents leads to a depolymerization of the polyphosphate chains, resulting in an increase of shorter chains species based on pyrophosphates. The identification and quantification of Ni2P4012, β-Ni2P2O7 and δ-Ni2P2O7 crystalline phases present in the nickel polyphosphate glass–ceramics were carried out by X-ray powder diffraction using the Rietveld method. Quantitative phase analyses of the three polymorph forms were also evaluated. Based on interpretation of reflectance spectroscopy and Rietveld refinement, Ni2+ ions may occupy distorted octahedral and tetrahedral sites.
1. Introduction
Phosphate glasses are interesting materials for technological and optical applications due to their low glass transition temperature (Tg), low melting point (Tm), broad transparency for ultraviolet (UV) light, when compared with silicate glasses, lower viscosity in the liquid state and higher thermal expansion coefficient than silicate and borate glasses.1–4 Phosphate glasses are successfully obtained from a coacervation route, which involves the interaction of a sodium polyphosphate Na(PO3)n solution (Graham salt) with mainly divalent ions, such as Ca2+, Na+ and Zn2+, and show a great potential to produce phosphate glasses as precursors for optical materials.5–8The preparation of polyphosphate coacervate glasses through coacervation route was also proposed in the study of Gomez et al., which allowed subsequent studies of glasses preparation using polyphosphates coacervates as precursors.5 Subsequently, Palavit et al. studied the method of glass preparation from coacervate based on zinc–sodium polyphosphate solutions into different pH values and the counter-ion nature.6 This study showed that the counter-ion nature of zinc salts can influence the coacervation process. Willot et al. studied the flexibility and versatility of the coacervate route, with or without the addition of methanol to the NaPO3–ZnX–H2O system, in which (X = Cl−, SO42− and NO3−), and the subsequent preparation of the glasses.7 Under this study, the glass composition and consequently its properties can be adjusted by parameters, such as the concentrations of sodium polyphosphate and zinc salt, as well as, the pH. Franco et al. highlighted the importance of polyphosphate coacervates to form the glass precursor in aqueous environment and at room temperature.8 Furthermore, it showed that the methanol addition to Na(PO3)n solution favors the coacervation process, by reducing the dielectric constant of the mixture and eliminating the solvation layer, consequently, allowing interaction between the polyphosphate chains.
Polyphosphate coacervates precursors are amorphous materials prepared by breaking the stabilization of the polyphosphate colloidal solutions by adding electrolytes or solvents with a dielectric constant lower than water, which is the polyphosphate solvent.9,10 The interaction among aqueous sodium polyphosphate Na(PO3)n solution with multivalent ions, such as Ca2+, Mn2+, Co2+, Ni2+, Fe3+, Eu3+ and Al3+ results in a phase separation process.11–16 The viscous phase, rich in colloids, is called coacervate, while the less viscous phase is the supernatant.9 In some cases, the coacervation process may be induced by the addition of a low molecular weight solvent, as proposed by Umegaki et al. in 1970.10 According to Umegaki et al., it is necessary the addition of 10% of methanol (v/v) into the sodium polyphosphate solution in order to obtain coacervates.
As previously studied by Silva et al.,11,16 polyphosphate coacervation process and spectroscopic properties of Co(II) and Ni(II) coacervates were prepared by addition of 10% (v/v) of methanol into the sodium polyphosphate solution. In the first study, the proposal and understanding of the coacervation process of Co2+ and Ni2+ coacervates was studied through X-ray Absorption Spectroscopy (EXAFS analysis) and Raman Spectroscopy.11 Meanwhile, in a second study, transparent amorphous materials, based in Co(II) and Ni(II) coacervates, were evaluated in terms of their structural and spectroscopic properties by X-ray diffraction, UV-VIS and Raman spectroscopic techniques. It is important to highlight that UV-VIS diffuse reflectance spectra from the Co(II) and Ni(II) coacervates, suggests the use of these materials as absorption filters.16 Besides, those materials had shown great potential for application in Agro-chemical industry,17,18 biomedical applications,19 pharmaceutical,20 and optical devices.21
In parallel, the X-ray diffraction is a key tool to characterize crystalline materials, as well as to identify and quantify different phases by refinement of the crystal structure using the Rietveld method.22 When the dimensions of the unit cell and space group are known, even if the crystal structure is unknown, it can be used for the refinement method Le Bail23 or Pawley24 for identification.
In this paper, the aim was study the influence of different low molecular solvents addition in the coacervation process of nickel polyphosphate coacervates (NiPC), and it subsequent uses for preparation of glass–ceramics (NiP-GC). NiPC was choose due to the lack of information on the using of different solvents with lower dielectric constant than water in order to start the coacervation process, such as ethanol (EtOH), dimethylketone (DMK), methanol (MeOH) and monoethylene glycol (MEG). Structural and spectroscopic properties of NiPC and NiP-GC were explored by differential scanning calorimeter (DSC), X-ray powder diffraction (XRD) analysis, Raman and UV-VIS spectroscopies.
2. Experimental
2.1 Coacervates preparation
Nickel polyphosphate coacervates (NiPC) were prepared by mixing 4 mol L−1 of Na(PO3)n solutions (Merck) to an equal volume of 2 mol L−1 NiCl2 (99.9%, Aldrich) solutions under constant stirring. Liquid–liquid phase separation was obtained by adding different solvents (ethanol, dimethylketone, methanol and monoethylene glycol) in a content of 10% v/v. The denser phase was frozen at −20 °C for 24 h and subsequently lyophilized using a L101 LIOTOP freezer-dryer during 12 h. After the lyophilizing process, the NiPC samples were kept into a vacuum desiccator containing silica-gel for further structural and spectroscopic investigations.2.2 Nickel polyphosphate glass–ceramics (NiP-GC)
NiP-GC systems were prepared by melting the dried NiPC as explained above in Section 2.1. Approximately 5 grams of NiPC for different solvents were obtained, and them NiPC and pure Na(PO3)n were melted in platinum crucibles at 1000 °C for 20 minutes and cast into pre-heated stainless steel molds. Table 1 shows the compositions and the label given for all NiPC and NiP-GC samples.| Samples | Solvents (10% v/v) | |
|---|---|---|
| NiPC | NiP-GC | |
| NiPC(EtOH) | NiP(EtOH) | Ethanol (EtOH) |
| NiPC(DMK) | NiP(DMK) | Dimethylketone (DMK) |
| NiPC(MeOH) | NiP(MeOH) | Methanol (MeOH) |
| NiPC(MEG) | NiP(MEG) | Monoethylene glycol (MEG) |
2.3 NiPC lyophilized and NiP glass–ceramics characterization
The structural and spectroscopic properties of the NiPC lyophilized and NiP-GC samples were investigated by using the following techniques: differential scanning calorimetry (DSC) measurements was carried out from 25 to 600 °C at a heating rate of 10 °C min−1, using a DSC Q600 equipment from TA Instruments under N2 (70 mL min−1) and estimate error is ± 2 °C for Tg and Tx. Raman scattering spectra were recorded at room temperature in a frequency range from 200 to 1500 cm−1 in a HORIBA Jobin Yvon model LabRAM HR micro Raman apparatus equipped with a 632.8 nm laser, delivering 17 mW. X-ray powder diffraction for the NiPC glass–ceramics were carried out with a Bruker D8 Advance diffractometer operating with a Ni filtered CuKα radiation source at 2θ angle ranging from 10 to 80° with a step pass of 0.02° and a step time of 2 s. All refinements was performed by TOPAS Academic v 5.0,25 it was used the referred crystalline structure described by Masse, R. et al. to phase delta,26 Pietraszko and Lukaszewicz (1968) to phase beta27 and Nord (1974) to phase Ni2P4O12.28 In the Rietveld method the background was fitted using the Chebyschev polynomial function with 8-terms, the peak profile was modeled by the fundamental parameters approach implemented in TOPAS Academic 5.0. Also unit cell of the phases was refined. Reflectance spectra of NiP-GC samples were obtained by using a VARIAN Cary 5000 UV-Vis-near infrared (NIR) spectrophotometer, in the spectral range from 200 to 800 nm.3. Results and discussion
3.1 Lyophilized nickel polyphosphate coacervates (NiPC)
Fig. 1(a) shows the Raman spectra of all NiPC lyophilized samples and Na(PO3)n precursor. Raman spectrum of Na(PO3)n shows two main vibrational modes assigned to the symmetrical stretching of P–O–P bridge νs(P–Ob) and symmetrical stretching of P–O terminal groups νs(P–Ot) at 684 and 1160 cm−1, respectively.29 The addition of Ni2+ ions into the Na(PO3)n solution shifts the νs(P–Ob) and νs(P–Ot) bands of NiPC lyophilized samples as shown in Fig. 1(a) and Table 2.| NiPC | νs(P–Ob) | νs(PO32−) | νs(PO4)3− | νs(CC) | δ(CH2) | νs(P–Ot) |
|---|---|---|---|---|---|---|
| NiPC(EtOH) | 696 | 1085 | 1165 | |||
| NiPC(DMK) | 696 | 1077 | 1164 | |||
| NiPC(MeOH) | 696 | 1076 | 1164 | |||
| NiPC(MEG) | 695 | 1076 | 1037 | 865 | 886 | 1164 |
| Na(PO3)n | 684 | 1160 |
Silva et al. and Dias Filho et al. showed that the transition metal ions are located inside the cagelike sites constituted by long polyphosphates chains.16,30 The increase of transition metal ions concentration, and consequently saturation of the cagelike sites, begins at the coacervation process through supramolecular interactions between adjacent polyphosphates chains.
Fig. 1(b) show the behavior of νs(P–Ob) and νs(P–Ot) vibrational modes intensities of different lyophilized nickel coacervates as a function of solvents with different dielectric constants, compared to pure Na(PO3)n. The shifting to higher wavenumber values of the νs(P–Ob) vibrational mode is reflected in the increase of P–Ob bond constant force, which can be understood by the presence of nickel ions inside the phosphate cages formed by the long polyphosphate chains surrounding the nickel ions. In addition, the lyophilizing process allows the reduction of the metal ions interaction with water molecules, leading to an increase of the electronic density of Ni–Ot bonds, and a consequent increase of the P–Ot bond strength. Furthermore, the Raman spectrum of the NiPC(MEG) showed four vibrational modes: νs(P–Ob), νs(P–Ot), νs(PO4)3− and νs(PO32−) at 695, 1164 cm−1, 1037 and 1074 cm−1, respectively. Such bands have been assigned to phosphate groups as shown in Table 2.16 Additionally, two bands are observed at 865 and 886 cm−1, corresponding to the vibrational modes assigned to νs(C–C) and δ(CH2) (rocking) as suggested by Matsuura et al.31
The Fig. 1(c) shows the behavior of the integrated area Aνs(P–Ob)/Aνs(P–Ot) ratio (where A is the areas above the bands) as a function of the solvent. The decrease of Aνs(P–O–P)/Aνs(P–Ot) ratio indicates the hydrolysis of P–O–P bonds containing in the polyphosphate chains, smaller polyphosphate chains could be formed and it also suggest a modification of P–O–P hydrolysis by formation of Qn (n = 0, 1). As can be seen in Franco et al., the 31P NMR results for the sodium polyphosphate coacervates (NaPC) showed that reducing the dielectric constant of solution by addition of a solvent with low molecular weight promotes the coacervation process, but will also break the long polyphosphate chains into short ones.8
Following the decreasing order of dielectric constant values, e.g. εMEG > εMeOH > εEtOH > εDMK, it can be observed that the hydrolysis of P–O–P bonds more evident in solvents with higher dielectric constant (MEG and MeOH) producing phosphates species of smaller chains, such as pyrophosphate and/or orthophosphate which are not stabilized inside the coacervates, Fig. 2.
 | ||
| Fig. 2 Hydrolysis process of polyphosphate chains of the NiPC lyophilized in pyrophosphates and orthophosphates species by the addition of different solvents. | ||
3.2 Nickel polyphosphate coacervates – glass–ceramics (NiP-GC)
Sodium polyphosphate coacervates (NaPC) obtained by coacervation route is a good precursor to prepare glass materials.8 In this work, Ni-based polyphosphate coacervates glass–ceramics were prepared by melting and quenching the NiPC coacervates prepared by different low molecular solvent. Usually, glass–ceramics are obtained by nucleation subsequent crystal growth processes using controlled thermal treatment, which allows obtain amorphous materials containing one or more crystalline phases.32Regardless the solvent used in the coacervation processes, all NiPC samples were obtained as a dense and green colored phase, whereas the glass–ceramics show a brownish color (Table 3).
The NiP-GC samples showed relative transparence for NiP(EtOH) and NiP(MeOH) samples, while NiP(DMK) and NiP(MEG) samples are opaque by naked eye (Table 3).
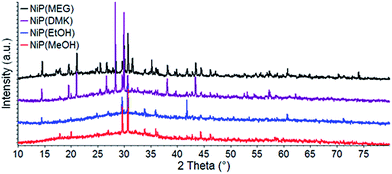
Moreover, thermal analysis measurements showed the presence of the glass transition phenomenon for all samples, confirming their glassy nature (Fig. 3). The glass–ceramic state of these samples was confirmed by the diffraction peaks over the diffuse halo (amorphous phase) (Fig. 4).
| Glass–ceramics | Tg (°C) | Tx (°C) | Tp (°C) | ΔT |
|---|---|---|---|---|
| NiP(EtOH) | 464 ± 2 | 506 ± 2 | 536 ± 2 | 42 |
| NiP(DMK) | 454 ± 2 | 499 ± 2 | 526 ± 2 | 45 |
| NiP(MeOH) | 435 ± 2 | 530 ± 2 | 571 ± 2 | 95 |
| NiP(MEG) | 306 ± 2 | 386 ± 2 | 422 ± 2 | 80 |
| Na(PO3)n – glass | 286 ± 2 | 302 ± 2 | 392 ± 2 | 16 |
During the synthesis, it was also used isopropanol alcohol as solvent in order to evaluate the influence of the chain size during the coacervation process. It was not possible to obtain coacervate materials, probably due to the long chain size of the organic solvent, which hump the approximation of the phosphate chains preventing the formation of the inorganic polymer skeleton. Table 4 summarizes the characteristic temperatures and the thermal stability against crystallization parameters obtained for all NiP-GC samples.
3.2.2.1 Structural refinement and quantification of crystalline phases by the Rietveld method. The Fig. 4 shows that all samples exhibit the halo, characteristic of amorphous material, and diffraction peaks. The identification and quantification of the crystal phases, with Rietveld method is summarized in the Table 5.
| Samples | Delta (%) | Ni2P4O12 (%) | Beta (%) |
|---|---|---|---|
| NiP(EtOH) | 91.3(8) | 8.7(8) | — |
| NiP(DMK) | 22.0(8) | 78.0(8) | — |
| NiP(MeOH) | 100 | — | — |
| NiP(MEG) | 49.3(6) | 42.8(5) | 7.9(4) |
Nickel polyphosphates, according to PDF-2 database, can crystallize like Ni2P2O7, but this structure shows three polymorphs: Ni2P4O12 (PDF-39-710), β-Ni2P2O7 (PDF75-1054) and δ-Ni2P2O7 (PDF 49-1082). All polymorphs can be easily differentiated by X-ray diffraction while another structure was found like Ni2P4O12 (86-2160). The bond angles and bond lengths for three polymorphs phases are displayed in the ESI.†
Percentage related to three polymorphs phases, obtained through the Rietveld Method is presented in Table 5. The quantitative phase analyses showed to presence the delta crystalline phase in different percentage (%) in all samples studied, and, in addition, the NiP(MEG) glass–ceramic presented all three polymorphs forms, resulting in delta 49.3(6) %, alpha 42.8(5) % and beta 7.9 (4) %, respectively. On the other hand, the NiP(MeOH) glass–ceramic has only the delta crystalline phase (100%) as more stable. Refinement quality indicators RWP (%) and Gof (goodness-of-fit) were indicated in the ESI.†
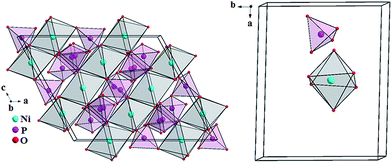
The crystal structure representations of three polymorphs Ni2P4O12, δ-Ni2P2O7 and β-Ni2P2O7 structure are displayed as polyhedral of atoms in Fig. 5, 6 and 7, respectively. The green, purple and red spheres correspond to the Ni2+, P5+ and O2− ions, respectively. Fig. 5 and 6 shows the units cells of Ni2P4O12 and δ-Ni2P2O7 crystalline phase where the Ni2+ ions occupy the (Ni–O) coordination sites of distorted octahedral arrangement and tetrahedral (PO4) structural units.
In the Fig. 7, the unit cell of β-Ni2P2O7 crystalline phase shows the Ni2+ ion in a tetrahedral arrangement assigned to distorted square planar geometry and tetrahedral (PO4) structural units. As will be discussed below, the UV-VIS spectroscopy data for all NiP-GC samples corroborate the X-ray diffraction data with respect to the coordination geometry of Ni2+ ions.
 | ||
| Fig. 9 (a) Raman spectra of NiP-GC samples, and Na(PO3)n glass obtained at 632.8 nm laser excitation and (b) Aνs(P–Ob)/Aνs(P–Ot) ratio as a function of the different solvents. | ||
| Glass–ceramics | νs(P–Ob) (Q2 units) | νs(PO3)2− (Q1 units) | νs(P–Ot) (Q2 units) |
|---|---|---|---|
| NiP(EtOH) | 696 | 1050 | 1172 |
| NiP(DMK) | 696 | 1050 | 1171 |
| NiP(MeOH) | 695 | 1050 | 1166 |
| NiP(MEG) | 684 | 1048 | 1151 |
| Na(PO3)n – glass | 691 | — | 1174 |
Fig. 9(b) shows the decrease of Aνs(P–Ob)/Aνs(P–Ot) ratio for all NiP-GC samples. In this case, the NiP(EtOH) has a lower modifier action of P–O–P hydrolysis on long polyphosphate chains and consequent formation of Q1 species. As previously shown in the Table 4 and Fig. 3(b), the glass transition temperature for NiP(EtOH) presents the highest value among NiP-GC samples analyzed. It is important to consider the relation between the glass transition temperature and the connectivity of the vitreous network. As showed, ethanol is the one that mostly contributes to preserve the vitreous network connectivity of the polyphosphate chains.
4. Conclusions
In this work, the nickel polyphosphates coacervates (NiPC) were prepared by direct addition of different solvents to nickel and sodium polyphosphate solutions. We have shown that the NiPC are good glassy precursors through of preparation of NiP-GC. The structural investigation of NiPC and NiP-GC by Raman spectroscopy showed that the solvent addition of low molecular weight promotes breaking the long polyphosphate chains to short chains assigned to pyrophosphates species. In addition, the identification and quantification of the crystalline phases in NiP-GC was verified by Rietveld method showed that NiP-GC samples can to crystallize in three polymorphs forms derived of nickel pyrophosphates, which corroborates with results of Raman spectroscopy. Besides, the Rietveld method showed that is a powerful tool used in the quantification process of crystalline phases in several types of materials, such as glass–ceramics.Acknowledgements
The authors are grateful to grants #2013/07793-6, São Paulo Research Foundation – FAPESP and CAPES-PNPD 2654/2011 for financial support.References
- R. K. Brow, J. Non-Cryst. Solids, 2000, 263&264, 1–28 CAS.
- M. Karabulut, E. Melnik, R. Stefan, G. K. Marasinghe, C. S. Ray, C. R. Kurkjian and D. E. Day, J. Non-Cryst. Solids, 2001, 288, 8–17 CrossRef CAS.
- N. Sharmin, C. D. Rudd, A. J. Parsons and I. Ahmed, J. Mater. Sci., 2016, 51, 7523–7535 CrossRef CAS.
- Y. Xiaoyan, D. E. Day, G. J. Long and R. K. Brow, J. Non-Cryst. Solids, 1997, 215, 21–31 CrossRef.
- F. Gomez and P. Vast, Phosphorus Res. Bull., 2000, 11, 61–68 CrossRef CAS.
- G. Palavit, L. Montagne and R. Delaval, J. Non-Cryst. Solids, 1995, 189, 277–282 CrossRef CAS.
- G. Willot, F. Gomez, P. Vast, V. Andries, M. Martines, Y. Messaddeq and M. Poulain, C. R. Chim., 2002, 5, 899–906 CrossRef CAS.
- D. F. Franco, H. S. Barud, S. Santagneli, R. S. Lamarca, B. F. Santos, M. A. P. Silva, L. F. C. de Oliveira, S. J. L. Ribeiro and M. Nalin, Mater. Chem. Phys., 2016, 180, 114–121 CrossRef CAS.
- T. Umegaki and T. Kanazawa, Bull. Chem. Soc. Jpn., 1973, 46, 3587–3588 CrossRef CAS.
- T. Umegaki, Y. Nakayama and T. Kanazawa, Bull. Chem. Soc. Jpn., 1976, 49, 2105–2107 CrossRef CAS.
- M. A. P. Silva, D. F. Franco and L. F. C. De Oliveira, J. Phys. Chem. A, 2008, 112, 5385–5389 CrossRef CAS PubMed.
- T. Umegaki and T. Kanazawa, Bull. Chem. Soc. Jpn., 1979, 52, 2124–2126 CrossRef CAS.
- L. F. C. De Oliveira, M. A. P. Silva, A. R. Brandão, R. Stephani, C. I. R. De Oliveira, R. R. Gonçalves, A. J. Barbosa, H. S. Barud, Y. Messaddeq and S. J. L. Ribeiro, J. Sol-Gel Sci. Technol., 2009, 50, 158–163 CrossRef.
- N. C. Masson, E. F. De Souza and F. Galembeck, Colloids Surf., A, 1997, 121, 247–255 CrossRef CAS.
- E. C. O. Lima, J. M. M. Neto, Y. F. Fujiwara and F. Galembeck, J. Colloid Interface Sci., 1995, 176, 388–396 CrossRef CAS.
- M. A. P. Silva, D. F. Franco, A. R. Brandão, H. S. Barud, F. A. Dias Filho, S. J. L. Ribeiro, Y. Messaddeq and L. F. C. De Oliveira, Mater. Chem. Phys., 2010, 124, 547–551 CrossRef CAS.
- Y. Zhu, X. Na and S. Li, J. Surfactants Deterg., 2009, 12, 305–311 CrossRef CAS.
- Y. Yan, E. Kizilay, D. Seeman, S. Flanagan, P. L. Dubin, L. Bovetto, L. Donato and C. Schmiitt, Langmuir, 2013, 29, 15614–15623 CrossRef CAS PubMed.
- D. M. Pickup, R. J. Newport, E. R. Barney, J. Kim, S. P. Valappil and J. C. Knowles, J. Biomater. Appl., 2014, 28, 1226–1234 CrossRef CAS PubMed.
- N. Shahgholian and G. Rajabzadeh, Food Hydrocolloids, 2016, 59, 17–25 CrossRef CAS.
- F. A. D. Filho, S. J. L. Ribeiro, R. R. Gonçalves, Y. Messaddeq, L. D. Carlos, V. Z. Bermudez and J. Rocha, J. Alloys Compd., 2004, 374, 74–78 CrossRef.
- H. M. Rietveld, Acta Crystallogr., 1966, 21, A229 Search PubMed.
- A. Le Bail, Powder Diffr., 2005, 20, 316–326 CrossRef CAS.
- G. S. Pawley, J. Appl. Crystallogr., 1981, 14, 357–361 CrossRef CAS.
- A. Coelho, Coelho Software: TOPAS Academic v. 5.0, Topas Academic, Brisbane, 2007 Search PubMed.
- R. Masse and J. C. Guitel, et al., Mater. Res. Bull., 1979, 14, 337–341 CrossRef CAS.
- A. Pietraszko and K. Lukaszewicz, Sciences Chimiques, 1968, 16, 183–187 CAS.
- A. G. Nord, Acta Chem. Scand., Ser. A, 1974, 28, 539–543 Search PubMed.
- C. I. R. De Oliveira, L. F. C. De Oliveira, F. A. Dias Filho, Y. Messaddeq and S. J. L. Ribeiro, Spectrochim. Acta, Part A, 2005, 61, 2023–2028 CrossRef PubMed.
- F. A. Dias Filho, L. D. Carlos, Y. Messaddeq and S. J. L. Ribeiro, Langmuir, 2005, 21, 1776–1783 CrossRef CAS PubMed.
- H. Matsuura, M. Hiraishi and T. Miyazawa, Spectrochim. Acta, Part A, 1972, 28, 2299–2304 CrossRef CAS.
- R. C. Ropp, Elsevier, 1992, 15, 43 Search PubMed.
- S. P. Tandon and J. P. Gupta, J. Solid State Chem., 1970, 2, 283–284 CrossRef CAS.
- C. K. Jorgensen, Absorption Spectra and Chemical Bonding in Complexes, Pergamon Press, Oxford, 1962 Search PubMed.
- D. S. Mcclure, Electronic Spectra of Molecules and Ions in Crystals, Academic Press, Inc., New York, 1959 Search PubMed.
- Y. Tanabe and S. Sugano, J. Phys. Soc. Jpn., 1954, 9, 753–766 CrossRef CAS.
- V. P. Solntsev, E. V. Pestryakov, A. I. Alimpiev, E. G. Tsvetkov, V. N. Matrosov, V. I. Trunov and V. V. Petrov, Opt. Mater., 2003, 24, 519 CrossRef CAS.
- N. V. Kuleshov, V. G. Shcherbytsky, V. P. Mykhailov, S. Kück, J. Koetke, K. Petermann and G. Huber, J. Lumin., 1997, 71, 265 CrossRef CAS.
- Y. M. Moustafa and K. El-Egili, J. Non-Cryst. Solids, 1998, 240, 144–153 CrossRef CAS.
- Y. M. Lai, X. F. Liang, S. Y. Yang, J. X. Wang and B. T. Zhang, J. Mol. Struct., 2012, 1013, 134–137 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra20696d |
| This journal is © The Royal Society of Chemistry 2016 |