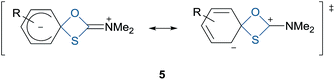Open Access Article
Open Access ArticleMicrowave-mediated Newman–Kwart rearrangement in water†
Ina
Hoffmann
and
Jürgen
Schatz
*
Friedrich-Alexander University Erlangen-Nürnberg, Department of Organic Chemistry I, Henkestraße 42, 91054 Erlangen, Germany. E-mail: juergen.schatz@fau.de; Fax: +49 9131 85 24707; Tel: +49 9131 85 25766
First published on 19th August 2016
Abstract
For the first time the unimolecular Newman–Kwart rearrangement is performed in pure water. The elevated temperatures required for the 1,3-aryl shift are easily accomplished by microwave irradiation. Differently functionalized substrates underline the expected influence on the reaction rate regarding the electronic and steric effects of the substituents. The utilization of supramolecular additives enables an increase in conversion. Besides, the conduction in a microwave reactor allows for a rapid collection of kinetic data affording the reaction rate constants of the rearrangement of distinct substrates as well as the Arrhenius constant and activation parameters of the conversion of 2- and 4-nitrophenyl-O-thiocarbamate.
Introduction
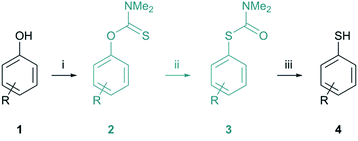
The thermally induced reaction of O-arylthiocarbamates to their corresponding S-arylthiocarbamates is commonly known as the Newman–Kwart rearrangement (NKR) and was first discovered in the 1960s.1–3 In light of the easy availability of various phenols 1, their facile conversion to N,N-dimethyl O-arylthiocarbamates 2 as well as the readily achieved cleavage of the NKR product 3 by hydrolysis, methanolysis, or reduction, the NKR is one of the most synthetically attractive routes for the formation of thiophenols 4 from phenols 1 (Scheme 1).4–6 Both thiophenols and similarly accessible sulfur-containing compounds such as sulfoxides, sulfones, thioethers, and sulfonic acids contribute to a broad field of applications including agrochemicals, dyestuffs, medicinal chemistry, chiral ligands, molecular switches, organocatalysts, dendrimers and supramolecular assemblies.5–7 Not least, the NKR can be utilized for the synthesis of specific arenes from phenols 1 by desulfurization of the corresponding thiophenols 4.The NKR proceeds via first-order kinetics and is based on an intramolecular migration of an aryl moiety from an oxygen atom to a sulfur atom (OAr → SAr), which is thermodynamically driven by the formation of a strong carbon–oxygen double bond and the simultaneous cleavage of the weaker carbon–sulfur double bond.8 This 1,3-aryl shift can also be considered as an intramolecular nucleophilic aromatic substitution with sulfur attacking the ipso-carbon atom. The rearrangement proceeds via a zwitterionic four-membered cyclic transition state 5 (Fig. 1), requiring elevated temperatures in the range of 200–300 °C to accomplish the high activation energy (EA, ΔG‡).8 Electron withdrawing groups (EWG) attached to the negative cyclohexadienyl moiety facilitate the OAr → SAr migration and therefore, either reduce the reaction temperature or reaction time.9,10N,N-Dimethyl O-arylthiocarbamates 2 bearing one EWG in ortho position can be even more easily converted due to the rotational restriction around the aryl–oxygen bond, which entails a loss in entropy and the associated higher order in the transition state.11,12 On the downside, doubly ortho, sterically hindered, and electron donating substituents (EDG) decrease the reaction rate and therefore, require temperatures approaching 300 °C or prolonged reaction times.13 The general harsh reaction conditions not only cause undesired side reactions and charring, but also pose a safety issue for the organic chemist. Notably, Harvey et al. performed the NKR at only 100 °C yielding quantitative conversions for several N,N-dimethyl O-arylthiocarbamates 2 by means of [Pd(tBu3P)2] as catalyst.14 In addition to that, Perkowski et al. utilized a commercially available photooxidant, which efficiently promoted the NKR at ambient temperature.15 A convenient alternative to at least circumvent the safety issue stemming from high reaction temperatures is the conduction of the rearrangement in a microwave (MW) reactor under small-scale autoclaves conditions.7,8,13,16 Further advantages compared to the conventional heating in an oil-bath are for instance the rapid access to high reaction temperatures as well as the fast cooling with compressed air at the end of the reaction, improved purity profiles, energy efficient in-core volumetric heating of the reaction medium, and practicability in terms of handling, i.e., accurate control of reaction parameters and if necessary, the rapid collection of several data points needed for a kinetic study.16,17 Moseley et al. investigated the NKR under MW irradiation and found no substantial difference between conventional heating and MW radiation.7 However, for the first time a solvent rate effect was experimentally described, showing an enhancement of the conversion with increasing polarity of the solvent. This is not surprising considering the highly polar transition state 5, which should be stabilized by polar solvents, and therefore enhance the reaction rate with decreasing EA. Addressing green chemistry, now the question arises to what extent water, possessing a dielectric constant (εr) of 78, is able to stabilize the transition state 5.18
Over the last decades water has received increasing attention as solvent in organic synthesis and promoted an intriguingly diverse field of reactions ranging from pericyclic reactions over transition-metal catalysis to reactions with carbenes and radicals just to name a few.18–22 In addition to the obvious benefits towards organic solvents of being inexpensive, non-flammable, non-toxic, and readily available, in many cases water is able to enhance both the reaction rate and the reaction selectivity due to hydrophobic effects.23–25 One alternative way to increase the reaction rate and selectivity or rather overcome solubility issues of non-polar substrates in water, is the utilization of supramolecular additives such as cyclodextrines or calix[n]arenes, which encapsulate the hydrophobic reactants in their cavities by non-covalent interactions while being water-soluble themselves.26–33
However, to our knowledge the sheer NKR has so far not been performed in pure water.34 Notably, owing to the fact, that the NKR can be conducted neat,1,5,8,9,11,35–40 this paper does not claim more environmentally benign reaction conditions, but rather demonstrates the attempt to lower EA by means of the highly polar and green solvent water. In this context we decided to carry out the rearrangements in pure water by means of MW irradiation. Though water is known to be only a medium absorber (loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) = 0.123), it can be rapidly heated well above its boiling point in closed vessels and has already been successfully utilized in many microwave-assisted reactions.17,41,42
δ) = 0.123), it can be rapidly heated well above its boiling point in closed vessels and has already been successfully utilized in many microwave-assisted reactions.17,41,42
In this paper, we report the effect of differently functionalized substrates utilizing electron-donating as well as electron-withdrawing substituents, the conversion dependency with and without MW irradiation, the influence of supramolecular additives (cf.Fig. 3), i.e. cucurbit[6]uril, imidazolio- and sulfocalix[n]arenes, and finally, a more in-depth kinetic study of 2- and 4-nitrophenyl O-thiocarbamate investigating the effect of water in terms of a possible transition state 5 stabilization.
Results and discussion
Substrates and scope
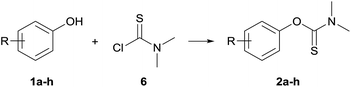
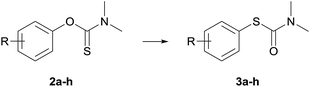
The substrates utilized for NKR, i.e., O-arylthiocarbamates 2a–h, were chosen to cover both steric and electronic effects on the rearrangement (Table 1). Electron-withdrawing nitro-groups substituted in ortho-2a, meta-2c, and para-position 2b of the arene should display the conversion dependency on the substitution pattern within one group regarding both steric and electronic characteristics, whereas further EWGs as well as EDGs substituted in para-position should be compared purely regarding their electronic influence. Besides, all O-arylthiocarbamates 2a–h as well as S-arylthiocarbamates 3a–h are known from literature and the results, i.e., conversion and reaction rates can be well correlated with Hammet σ constants.44| Entry | R | t [h] | Yield [%] | NKR substrate |
|---|---|---|---|---|
| a Reaction conditions: phenol 1 (1 eq.), DMTCC 6 (1.1 eq.), DABCO (1.3 eq.), NMP, 50 °C.7 b DMTCC 6 (1.2 eq.), DABCO (2.5 eq.), DMF, 65 °C.43 | ||||
| 1 | 2-NO2 | 3.8 | 92 | 2a |
| 2 | 4-NO2 | 2.5 | 91 | 2b |
| 3 | 3-NO2 | 5.8 | 93 | 2c |
| 4 | 4-CN | 3.0 | 88 | 2d |
| 5 | 4-COMeb | 1.5 | 96 | 2e |
| 6 | 4-Br | 2.5 | 84 | 2f |
| 7 | 4-F | 5.0 | 83 | 2g |
| 8 | 4-OMe | 3.7 | 59 | 2h |
The syntheses of the substrates proceeded in a single-step reaction starting from readily available, less acidic phenols 1a–h, DABCO and dimethylthiocarbamoyl chloride (DMTCC) 6 according to the procedures of Moseley and DeCollo.7,43 Direct precipitation by addition of water afforded products in good to excellent yields (59–96%), most of them being highly crystalline with the exception of substrates 2d (4-CN), 2g (4-F), and 2h (4-OMe), which were recrystallized from ethanol.
For our initial substrate screenings we decided to conduct the NKR of substrates 2a–h, all being completely insoluble in water at room temperature, at 180 °C and determine the conversions after 20 min (Table 2). At this temperature solely the rearrangements of 2-nitro 2a (>99%) and 4-nitrophenyl-O-thiocarbamate 2b (99%) resulted in quantitative and excellent yields. The less strongly electron-withdrawing substrates 2d (4-CN, 9%) and 2e (4-COMe, 15%) gave conversions being seven to eleven times lower, while the electronically disfavored O-arylcarbamates 2c (3-NO2) and 2f–h (4-Br, 4-F, 4-OMe) did not convert at all or not considerably (Table 2, Entry 6 2%). By raising the temperature over 200 °C to 210 °C, 220 °C for 3-nitrophenyl-O-thiocarbamates 2c respectively, the 3-nitro 2c, 4-fluoro 2g, and 4-methoxy 2h substrates remained unconverted and the rearrangement of 4-bromophenyl-O-thiocarbamate 2f increased by 2%. In our case a further enhancement of the temperature was not feasible due to the maximum limit of pressure (20 bar), which has almost been reached, and additionally, an integrated security limit of 250 °C of the applied microwave reactor. A quick solution might be the addition of inorganic salts in order to increase the transformation of microwave energy into heat, the utilization of larger reaction vessels or concentrations, and/or prolonged reaction times. On the other hand both electronically favored substrates 4-cyano-2d (9 → 46 → 81%) and 4-acetylphenyl-O-thiocarbamate 2e (15 → 42 → 64%) underwent a significant increase in conversion with rising temperature. On the whole the results of this coarse investigation are in good agreement with the substituent constants of Hammett (σ) also depicted in Table 2. However, the values of the nitro substrate substituted in meta-position 2c differ as expected, due to the insufficient stabilization of the negative cyclohexadienyl fragment in the transition state 5.
| Entry | R | σ | Conv. [%] at 180 °C | Conv. [%] at 200 °C | Conv. [%] at 210 °C |
|---|---|---|---|---|---|
| a Reaction conditions: O-arylthiocarbamate 2a–h (500 μmol, 0.2 M), MW irradiation, H2O (2.5 mL), 20 min. b Isolated yield in parentheses. c 220 °C. d Apart from conversion some product degradation occurred. | |||||
| 1 | 2-NO2 | — | > 99 (89)b | — | — |
| 2 | 4-NO2 | +0.778 | 99 | — | — |
| 3 | 3-NO2 | +0.710 | 0 | 0 | 0c |
| 4 | 4-CNd | +0.628 | 9 | 46 | 81 |
| 5 | 4-COMe | +0.516 | 15 | 42 | 64 |
| 6 | 4-Br | +0.232 | 2 | 3 | 4 |
| 7 | 4-F | +0.062 | 0 | 0 | 0 |
| 8 | 4-OMe | −0.268 | 0 | 0 | 0 |
As a result of our kinetic survey, which will be discussed later on, the rearrangement of 2-nitrophenyl-O-thiocarbamate 2a pointed out to be quantitative inside of 10 min at 180 °C (cf.Fig. 6). With this in hand we were interested in how MW irradiation affects the NKR in pure water. Therefore the rearrangement of substrate 2a was additionally conducted neat and in water for one using a pressure tube placed inside a heat-on plate and for another applying a conventional oil-bath and reflux condenser. Table 3 shows that the conversion decreased in the order microwave > neat ≫ pressure tube > reflux, unambiguously indicating a microwave effect for water. Under usual reflux conditions 2-nitrophenyl-O-thiocarbamate 2a did not convert at all, whereas a build-up of pressure slightly facilitated the reaction, which implies the conformational loss of freedom in the transition state and a negative volume of activation (Table 3, Entry 3 6%). With increasing temperatures the bond angle of water widens and its dielectric constant decreases. As a result, approaching temperatures greater or equal to 150 °C, water becomes increasingly non-polar, enhancing the ability to solubilize hydrophobic substrates.42,45 As compared to the MW-assisted rearrangement, water did not function as pseudo-organic solvent at the thermal experiments, where the NKR substrate 2a was barely dissolved. Therefore, the quantitative conversion by means of MW is probably due to the rapid achievement of the adjusted temperature and especially, an effective internal heating (in-core) resulting in a direct interaction of MW energy with solvent and substrate.
| Entry | Condition | Conversion [%] | k [s−1] | t 1/2 [s] |
|---|---|---|---|---|
| a Reaction conditions: 2-nitrophenyl-O-thiocarbamate 2a (500 μmol, 0.2 M), H2O (2.5 mL), 10 min, 180 °C. n/d = not determined. | ||||
| 1 | Microwave | >99 | 8.63 × 10−3 | 80.3 |
| 2 | Neat | 88 | 3.51 × 10−3 | 197 |
| 3 | Pressure tube | 6 | n/d | n/d |
| 4 | Reflux | 0 | n/d | n/d |
The solvent-free conduction yielded a conversion of 88% after 10 min at 180 °C. Besides, the determined reaction rate constants (k) reveal the neat reaction to be about two and a half times slower than the MW-mediated rearrangement.
Overall, the usage of MW energy and water as reaction medium for the NKR appears to be an effective combination as has already been shown in many reactions before.41,42 In addition to that, this method is generally beneficial in terms of easy workup and safe reaction control.
Supramolecular additives
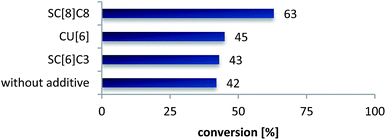
In recent studies we could show that supramolecular additives such as sulfocalix[n]arenes can efficiently increase the catalytic activity of Suzuki and metathesis reactions performed in pure water.27,46 The ability to solubilize hydrophobic reactants and an improved mass transfer are characteristics, which make water-soluble calix[n]arenes valuable for reactions in aqueous media or neat water. This tempted us to investigate the influence of macrocyclic additives on the NKR. Therefore we chose the rearrangement of 4-acetylphenyl-O-thiocarbamate 2e at 200 °C in 20 min (42% conversion, cf.Table 2) and added three different host compounds at a concentration of 0.5 mol% (Fig. 2 and 3). The reactions with cucurbit[6]uril 8 and SC6C3 7a resulted in hardly increased conversions of 43% and 45%, respectively, whereas the employment of SC8C8 7b gave an 21% improvement versus the rearrangement without additive. As described above, at elevated temperatures water acts as pseudo-organic solvent and is able to dissolve non-polar substrates. Owing to the fact that the substance probe is automatically cooled with compressed air after the reaction and thus, the organic product precipitated from water at ambient temperature, it is not detectable if the reactant had been fully dissolved afore. Therefore, we assume that SC8C8 7b contributes to an additional stabilization of the transition state 5 and an either fully or faster solubilization by encapsulating the hydrophobic NKR substrate in its cavity. A possible reason for the conversion difference of the two sulfocalix[n]arenes 7a, b may be an enhanced surface-activity of SC8C8 7b due to its larger hydrophobic tails. In general, the increased conversion might also derive from an enlarged loss tangent, i.e. the addition of salts, in our case 0.5 mol% SC8C8 7b, improves the MW absorbance due to ionic conduction.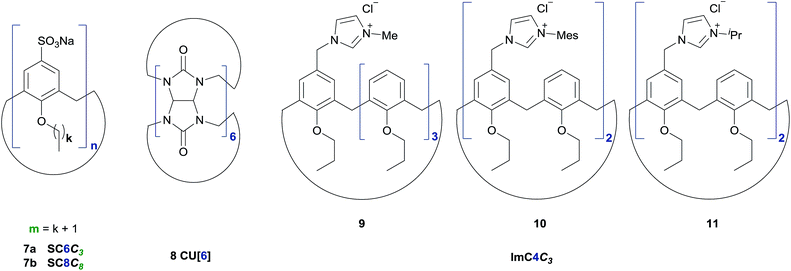 | ||
| Fig. 3 Used supramolecular additives for the NKR in pure water (left: sulfocalix[n]arenes 7a–b, middle: cucurbit[6]uril 8, right: imidazolio calix[4]arenes 9–11). | ||
In a second experiment, cationic imidazolio calix[4]arenes (9–11) were employed to possibly stabilize the transition state 5 of the NKR of 3-nitrophenyl-O-thiocarbamate 2c at 180 °C (cf.Fig. 3). However, none of the cations initiated the rearrangement, which underlines that the electronically disfavored 3-nitro substrate 2c requires higher temperatures to overcome the activation barrier.
Kinetic study
| [A]t = [A]0 × e−kt | (1) |
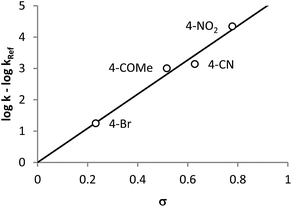
As described in literature,1,7,9,12,16 substituents differing in their electronic characteristics, have a strong impact on the reactivity of the NKR and additionally, are in good agreement with the Hammett σ constants. This correlation could also be seen for the rearrangement in pure water (vide supra) and should now be further investigated regarding the corresponding reaction rates. For this purpose, several NKRs of para-substituted substrates, i.e. nitro-2b, cyano-2d, acetyl-2e, and bromophenyl-O-thiocarbamate 2f, were conducted at 200 °C. The rate constant of the substrate bearing the nitro group in ortho position 2a was extrapolated from measurements at 140–180 °C using eqn (2) (cf.Fig. 8). Fig. 4 shows the exponential curves of the respective reactants displaying a reaction rate enhancement in the order 2-nitro > 4-nitro ≫ 4-cyano > 4-acetyl ≫ 4-bromo, which is still in line with the substituent constants of Hammett and former literature (cf.Fig. 5). The σ values, the corresponding rate constants, and the half-lives are depicted in Table 4. The rearrangement of substrate 2a is additionally promoted due to the steric restriction in the ortho position and would be completely converted in about three minutes. As opposed to that, the quantitative rearrangement of 4-bromophenyl-O-thiocarbamate 2f would be feasible within five days.
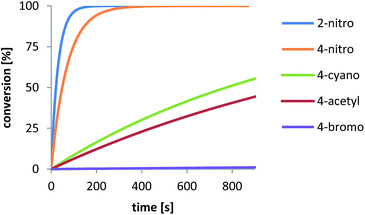 | ||
| Fig. 4 Dependency of conversion on time of the NKR of distinct substrates at 200 °C. Solid lines indicate fit-values calculated from rate constants in Table 4. | ||
| Entry | R | k [s−1] | t 1/2 [s] | σ |
|---|---|---|---|---|
| a Extrapolated from measurements at 140–180 °C using k = 4.06 × 1012 s−1 × exp(−128 kJ mol−1/R × 473.15 K) (cf.Fig. 7). | ||||
| 1 | 2-NO2 | 3.30 × 10−2a | 21.0 | — |
| 2 | 4-NO2 | 1.42 × 10−2 | 48.8 | +0.778 |
| 3 | 4-CN | 8.95 × 10−4 | 774 | +0.628 |
| 4 | 4-COMe | 6.51 × 10−4 | 1.07 × 103 | +0.516 |
| 5 | 4-Br | 1.15 × 10−5 | 6.04 × 104 | +0.232 |
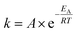 | (2) |
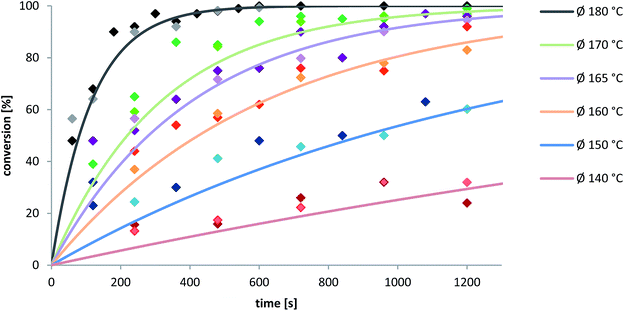 | ||
| Fig. 6 Dependency of the conversion on time of the rearrangement of 2-nitro-O-thiocarbamate 2a at temperatures ranging from 140 to 180 °C. Points indicate the experimental data from two measurements and the solid lines are the fit-values calculated using the averaged reaction rates (cf.Fig. 7). Averaged standard deviation of Ø k-values is 2.92 × 10−4 s−1. | ||
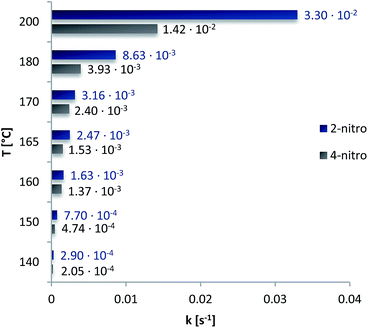 | ||
| Fig. 7 Averaged rate constants of 2-nitrophenyl-O-thiocarbamate 2a and rate constants of 4-nitrophenyl-O-thiocarbamate 2b at temperatures ranging from 140–200 °C. The reaction rate constants of substrate 2a at 200 °C and substrate 2b at 165 °C were extrapolated using eqn (2). | ||
As was expected, for one the reaction rates increase with higher temperatures and for another the beneficial steric acceleration of the ortho substrate 2a is mirrored in the reaction rates, being lower for the para substrate 2b at every temperature.
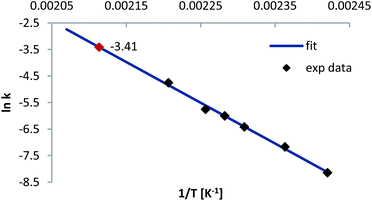
For both carbamates the Arrhenius constant (A) and EA were determined by plotting the k-values according to eqn (3) (cf.Fig. 8 for 2-NO2 substrate 2a) and the activation parameters, i.e. activation enthalpy (ΔH‡) and activation entropy (ΔS‡), were calculated using eqn (4) and (5):
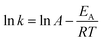 | (3) |
| ΔH‡ = EA − RT | (4) |
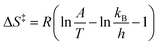 | (5) |
Table 5 shows the results for both systems. EA and the activation enthalpy, which is smaller by the product of RT, are each higher for the 2-NO2 substrate 2a. Moreover, the comparison with the values of Gilday et al., who performed the NKR of 2-nitrophenyl-O-thiocarbamate in poorly polar dichlorobenzene, even reveals the EA in water to be higher by the factor of 12 kJ mol−1 diminishing the assumption of a possible transition state 5 stabilization.16 On the contrary, comparing our results of the rearrangement of 4-nitrophenyl-O-thiocarbamate 2b with literature, where the NKR has also been conducted in a highly polar solvent, i.e. dimethylacetamide, the activation barrier could be reduced by approximately 11 kJ mol−1 in pure water.
The evaluation of the ΔS‡-values is not as straightforward. On the one hand the entropy of activation is increasing with extending reaction rate constant. This correlation is given in the Eyring eqn (6) and is also in line with our obtained results for the ortho-2a and para-nitro substrate 2b. Similar ΔS‡-correlations had been found for the NKR of 2- and 4-methylphenyl-O-thiocarbamate, which was ascribed to the restriction of the free rotation around the carbon–oxygen bond in the ground state.12
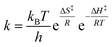 | (6) |
On the other hand, the activation entropy decreases with higher order in the transition state, i.e. loss of rotational and translational degrees of freedom, as compared to the initial state and becomes more negative with higher restriction. However, this conflicts with the lower negative activation entropy and the accelerated reaction rate constant of the sterically more restricted 2-nitrophenyl-O-thiocarbamate 2a.
Experimental section
General
Chemicals were purchased from commercial sources and used without further purification. O-Arylthiocarbamates 2a–h were prepared according to the published procedure.7,43 Imidazolio calix[4]arenes 9–11 were utilized by the courtesy of Dr. Markus Frank47 and alkylated sulfocalix[n]arenes 7a, b were utilized by the courtesy of Silvia Onodi.48 Maldi-ToF spectra were recorded on a Shimadzu Biotech AXIMA Confidence and EI spectra were recorded on a Finnigan MAT 95 XP from Thermo Scientific. IR spectroscopy was performed on a Varian 660-IR spectrometer. NMR spectra were recorded in CDCl3 using a Bruker Avance 400 operating at 400.13 MHz for 1H-NMR and 100.62 MHz for 13C-NMR. Chemical shifts (δ) are indicated in parts per million (ppm) relative to the internal standard (δH = 7.24 ppm, δC = 77.23 ppm). The conversions of the Newman–Kwart rearrangements were determined by comparing the integrated proton signals of the methyl groups of O-2a–h and S-aryl-N,N-dimethylthiocarbamate 3a–h. All conventional NKRs (with exception of the solvent-free experiment) were stirred at highest rate using a MR Hei-Tec from Heidolph®.Microwave-mediated Newman–Kwart rearrangement
The microwave-assisted reactions were performed employing an Initiator+ from Biotage® (fourth generation microwave synthesizer). For a general procedure, O-aryl-N,N-dimethylthiocarbamate (500 μmol) and H2O (2.5 mL) were placed in a sealed 2–5 mL vial. The reactant was stirred for a fixed time of 30 s and subsequently heated to the selected temperature not exceeding 400 W. All data on the reaction time exclude the heating time. After the sample was stirred at the respective temperature and time, it was automatically cooled down to approximately 50 °C and was afterwards allowed to further cool down to room temperature. The product was extracted with CDCl3 (3 × 600 μL) and subsequently used for 1H-NMR analysis.Preparative Newman–Kwart rearrangement
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (cm−1) = 1667 (s, N,N-disubstituted amide, νC
(cm−1) = 1667 (s, N,N-disubstituted amide, νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1590 (w, aryl, νC
O), 1590 (w, aryl, νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1570 (w, aryl, νC
C), 1570 (w, aryl, νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1461 (m, CH3, δC–H), 1352 (s, NO2, νN
C), 1461 (m, CH3, δC–H), 1352 (s, NO2, νN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 739 (m, aryl, νC–H); further absorption bands: 1524 (s), 1406 (w), 1300 (w), 1254 (m), 1092 (s), 1054 (m), 902 (m), 852 (m), 781 (m), 724 (m), 682 (m), 650 (m), 524 (w), 434 (w); 1H-NMR (400.13 MHz, CDCl3, 298 K): δH (ppm) = 2.98 (s, 3H), 3.08 (s, 3H), 7.49 (t, J = 7.7 Hz, 1H), 7.54 (t, J = 7.6 Hz, 1H), 7.67 (d, J = 7.7 Hz, 1H), 7.90 (d, J = 7.9 Hz, 1H); 13C-NMR (100.62 MHz, CDCl3, 298 K): δC (ppm) = 37.26, 124.45, 124.93, 130.03, 132.38, 138.32, 152.49, 164.51; MS (Maldi-ToF, dhb): calc. for C9H10N2O3S: 226.04, found: m/z = 101 [M − C2H22˙ + 2H˙+], 181 [M − NO2 + H+], 227 [M + H+].
O), 739 (m, aryl, νC–H); further absorption bands: 1524 (s), 1406 (w), 1300 (w), 1254 (m), 1092 (s), 1054 (m), 902 (m), 852 (m), 781 (m), 724 (m), 682 (m), 650 (m), 524 (w), 434 (w); 1H-NMR (400.13 MHz, CDCl3, 298 K): δH (ppm) = 2.98 (s, 3H), 3.08 (s, 3H), 7.49 (t, J = 7.7 Hz, 1H), 7.54 (t, J = 7.6 Hz, 1H), 7.67 (d, J = 7.7 Hz, 1H), 7.90 (d, J = 7.9 Hz, 1H); 13C-NMR (100.62 MHz, CDCl3, 298 K): δC (ppm) = 37.26, 124.45, 124.93, 130.03, 132.38, 138.32, 152.49, 164.51; MS (Maldi-ToF, dhb): calc. for C9H10N2O3S: 226.04, found: m/z = 101 [M − C2H22˙ + 2H˙+], 181 [M − NO2 + H+], 227 [M + H+].
Conventional Newman–Kwart rearrangements
Conclusions
In conclusion, we have shown that the microwave-mediated Newman–Kwart rearrangement (NKR) can be successfully performed in pure water. Substrates bearing electron-withdrawing substituents could be converted within short reaction times, whereas electron-donating groups demonstrated the limit of the reaction in water due to the immense pressure build-up with increasing temperature needed for a rearrangement. While this issue is a topic of current investigation, a possible answer was given by sulfocalix[n]arenes, whose addition facilitated the reaction affording higher conversions. Notably, based on our kinetic survey, a transition state stabilization could be ascribed to the reaction of the 4-NO2 carbamate and in addition, the reaction rates of the NKR of the 2-NO2 carbamate exceeded those of some commonly used organic solvents. Finally, control studies revealed a conclusive microwave (MW) effect for the rearrangement in water plus higher reaction rates than the solvent-free NKR.The fusion of MW irradiation and water, as environmentally benign reaction medium, not only affords quantitative Newman–Kwart rearrangements, but simultaneously meets two key principles of green chemistry, i.e. “energy-efficient synthesis” and “safer solvents”.49 The transfer to a pilot or production scale is an important topic, which has to be studied in future.
Acknowledgements
Generous support of the “Solar Technologies go Hybrid” (SolTech) initiative initiated by the Government of Bavaria is gratefully acknowledged.References
- M. S. Newman and H. A. Karnes, J. Org. Chem., 1966, 31, 3980–3984 CrossRef CAS.
- H. Kwart and E. R. Evans, J. Org. Chem., 1966, 31, 410–413 CrossRef CAS.
- J. D. Edwards and M. Pianka, J. Chem. Soc., 1965, 7338–7344 RSC.
- W. Walter and K.-D. Bode, Angew. Chem., Int. Ed., 1967, 6, 281–293 CrossRef CAS.
- G. Lloyd-Jones, J. Moseley and J. Renny, Synthesis, 2008, 661–689 CAS.
- C. Zonta, O. De Lucchi, R. Volpicelli and L. Cotarca, Top. Curr. Chem., 2007, 275, 131–161 CrossRef CAS PubMed.
- J. D. Moseley, R. F. Sankey, O. N. Tang and J. P. Gilday, Tetrahedron, 2006, 62, 4685–4689 CrossRef CAS.
- M. Burns, G. C. Lloyd-Jones, J. D. Moseley and J. S. Renny, J. Org. Chem., 2010, 75, 6347–6353 CrossRef CAS PubMed.
- K. Miyazaki, Tetrahedron Lett., 1968, 9, 2793–2798 CrossRef.
- A. Kaji, Y. Araki and K. Miyazaki, Bull. Chem. Soc. Jpn., 1971, 44, 1393–1399 CrossRef CAS.
- A. Mondragón, I. Monsalvo, I. Regla and I. Castillo, Tetrahedron Lett., 2010, 51, 767–770 CrossRef.
- H. M. Relles and G. Pizzolato, J. Org. Chem., 1968, 33, 2249–2253 CrossRef CAS.
- J. D. Moseley and P. Lenden, Tetrahedron, 2007, 63, 4120–4125 CrossRef CAS.
- J. N. Harvey, J. Jover, G. C. Lloyd-Jones, J. D. Moseley, P. Murray and J. S. Renny, Angew. Chem., Int. Ed., 2009, 48, 7612–7615 CrossRef CAS PubMed.
- A. J. Perkowski, C. L. Cruz and D. A. Nicewicz, J. Am. Chem. Soc., 2015, 137, 15684–15687 CrossRef CAS PubMed.
- J. P. Gilday, P. Lenden, J. D. Moseley and B. G. Cox, J. Org. Chem., 2008, 73, 3130–3134 CrossRef CAS PubMed.
- C. O. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250–6284 CrossRef CAS PubMed.
- A. Lubineau, J. Augé and Y. Queneau, Synthesis, 1994, 741–760 CrossRef CAS.
- C.-J. Li and L. Chen, Chem. Soc. Rev., 2006, 35, 68–82 RSC.
- U. M. Lindström, Organic Reactions in Water, Blackwell Publishing, Singapore, 2007 Search PubMed.
- C.-J. Li and T.-H. Chan, Comprehensive Organic Reactions in Aqueous Media, John Wiley & Sons, Inc., Hoboken, 2nd edn, 2007 Search PubMed.
- S. Kobayashi, Science of Synthesis: Water in Organic Synthesis, Georg Thieme Verlag, Stuttgart, 2nd edn, 2012 Search PubMed.
- M. Seßler and J. Schatz, Chem. Unserer Zeit, 2012, 46, 48–59 CrossRef.
- I. Hoffmann and J. Schatz, Nachr. Chem., 2013, 61, 748–753 CrossRef CAS.
- R. Breslow, Acc. Chem. Res., 1991, 24, 159–164 CrossRef CAS.
- M. Baur, M. Frank, J. Schatz and F. Schildbach, Tetrahedron, 2001, 57, 6985–6991 CrossRef CAS.
- I. Hoffmann, B. Blumenröder, S. Onodi née Thumann, S. Dommer and J. Schatz, Green Chem., 2015, 17, 3844–3857 RSC.
- F. Hapiot, J. Lyskawa, H. Bricout, S. Tilloy and E. Monflier, Adv. Synth. Catal., 2004, 346, 83–89 CrossRef CAS.
- S. Shimizu, T. Suzuki, Y. Sasaki and C. Hirai, Synlett, 2000, 1664–1666 CAS.
- D. Kirschner, M. Jaramillo, T. Green, F. Hapiot, L. Leclercq, H. Bricout and E. Monflier, J. Mol. Catal. A: Chem., 2008, 286, 11–20 CrossRef CAS.
- E. Monflier, G. Fremy, Y. Castanet and A. Mortreux, Angew. Chem., Int. Ed., 1995, 34, 2269–2271 CrossRef CAS.
- S. Shimizu, S. Shirakawa and Y. Sasaki, Angew. Chem., Int. Ed., 2000, 34, 1256–1259 CrossRef.
- T. Brendgen, T. Fahlbusch, M. Frank, D. T. Schühle, M. Seßler and J. Schatz, Adv. Synth. Catal., 2009, 351, 303–307 CrossRef CAS.
- N. Lal, L. Kumar, A. Sarswat, S. Jangir and V. L. Sharma, Org. Lett., 2011, 13, 2330–2333 CrossRef CAS PubMed.
- V. Albrow, K. Biswas, A. Crane, N. Chaplin, T. Easun, S. Gladiali, B. Lygo and S. Woodward, Tetrahedron: Asymmetry, 2003, 14, 2813–2819 CrossRef CAS.
- S. Cossu, O. De Lucchi, D. Fabbri, G. Valle, G. F. Painter and R. A. J. Smith, Tetrahedron, 1997, 53, 6073–6084 CrossRef CAS.
- D. Fabbri, G. Delogu and O. De Lucchi, J. Org. Chem., 1993, 58, 1748–1750 CrossRef CAS.
- M. Hori, T. Kataoka, H. Shimizu, M. Ban and H. Matsushita, J. Chem. Soc., Perkin Trans. 1, 1987, 1, 187 RSC.
- V. Novakova, M. Miletin, T. Filandrová, J. Lenčo, A. Růžička and P. Zimcik, J. Org. Chem., 2014, 79, 2082–2093 CrossRef CAS PubMed.
- R. Romagnoli, P. G. Baraldi, M. D. Carrion, O. Cruz-Lopez, M. Tolomeo, S. Grimaudo, A. Di Cristina, M. R. Pipitone, J. Balzarini, A. Brancale and E. Hamel, Bioorg. Med. Chem., 2010, 18, 5114–5122 CrossRef CAS PubMed.
- V. Polshettiwar, R. S. Varma, V. Cadierno, P. Crochet, S. E. García-Garrido, A. Fihri, C. Len, H. Zhao, C. Marestin, R. Mercier and B. Baruwati, Aqueous Microwave Assisted Chemistry: Synthesis and Catalysis, RSC Publishing, Cambridge, 2010 Search PubMed.
- D. Dallinger and C. O. Kappe, Chem. Rev., 2007, 107, 2563–2591 CrossRef CAS PubMed.
- T. V. DeCollo and W. J. Lees, J. Org. Chem., 2001, 66, 4244–4249 CrossRef CAS PubMed.
- R. W. Hoffmann, Aufklärung von Reaktionsmechanismen, Georg Thieme Verlag, Stuttgart, 1976 Search PubMed.
- C. R. Strauss and R. S. Varma, Top. Curr. Chem., 2006, 266, 199 CrossRef CAS.
- J. Tomasek, M. Seßler, H. Gröger and J. Schatz, Molecules, 2015, 20, 19130–19141 CrossRef CAS PubMed.
- M. Frank, Imidazolium-substituierte calix[4]arene: von molekularen Rezeptoren zu stabilen carbenen und Kreuzkupplungsreaktionen, translated “Imidazolio substituted calix[4]arenes: from molecular receptors to stable carbenes and cross-coupling reactions”, University Ulm, 2004 Search PubMed.
- S. Shinkai, S. Mori, H. Koreishi, T. Tsubaki and O. Manabe, J. Am. Chem. Soc., 1986, 108, 2409–2416 CrossRef CAS PubMed.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra20676j |
| This journal is © The Royal Society of Chemistry 2016 |