Exonuclease III-assisted substrate fragment recycling amplification strategy for ultrasensitive detection of uranyl by a multipurpose DNAzyme†
Abstract
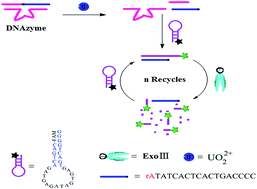
We report on a novel and highly sensitive strategy for UO22+ detection. The designed single-labeled hairpin signal probe (SSP) was employed as a reporter, and a multipurpose RNA-cleaving DNAzyme as a target recognition element, catalytic DNAzyme and the primer of substrate fragment recycling amplification. The presence of UO22+ results in the substrate strand in DNAzyme being cleaved at the RNA site, releasing short substrate fragments. The fragment that is complementary partially with SSP could open the hairpin of SSP to form a double-stranded DNA with recessed 3′-termini, which could be selectively digested by exonuclease III. After the double-stranded DNA is fully consumed, the substrate fragment is released. This one could hybridize with another SSP to initiate the next round of digestion by exonuclease III. The recycling of substrate fragments can be repeated multiple times, resulting in the accumulation of the more 6-carboxyfluorescein in the solution along with an amplified fluorescence signal. By this strategy, we achieved hitherto an unreported UO22+ detection limit of 2.4 pM. Therefore, this novel exonuclease III-amplified strategy has great potential for highly sensitive and selective quantification of UO22+ in biomedical research and environmental science fields.

 Please wait while we load your content...
Please wait while we load your content...