Ultrafast mechanochemical synthesis of copper sulfides†
Matej Baláž*a,
Anna Zorkovskáa,
Farit Urakaevb,
Peter Baláža,
Jaroslav Briančina,
Zdenka Bujňákováa,
Marcela Achimovičováac and
Eberhard Gockc
aDepartment of Mechanochemistry, Institute of Geotechnics, Slovak Academy of Sciences, Watsonova 45, 04001 Košice, Slovakia. E-mail: balazm@saske.sk; Tel: +421 557922603
bV S Sobolev Institute of Geology and Mineralogy SB RAS, Acad. Koptyug Av. 3, 630090 Novosibirsk, Russia
cInstitute of Mineral and Waste Processing, Waste Disposal and Geomechanics, Clausthal University of Technology, Walther-Nernst-Strasse 9, 38678 Clausthal-Zellerfeld, Germany
First published on 5th September 2016
Abstract
Covellite, CuS and chalcocite, Cu2S were prepared within a few seconds by ball milling of the elemental precursors. The morphology of the used copper, related to its preparation method, was found to be the key factor for the ultrafast reaction. The explosive character of the reaction was monitored by the gas pressure changes in the milling vessel and the reaction progress was pursued by X-ray diffraction analysis and Soxhlet's extraction. The local temperature at the contact site between the milling media and the milled mixture at the time of explosion was calculated as 950 °C for CuS and 700 °C for Cu2S. The mean crystallite size of the prepared products was 15 nm for CuS and 65 nm for Cu2S.
1. Introduction
The synthesis of semiconductor nanomaterials is of high interest these days. Among various candidates, copper sulfide has an inevitable place, as it is suitable for a wide spectrum of applications,1 namely for photocatalysis,2 solar cells,3 energy-storage devices4 and biomedical applications.5 The Cu–S system is of particular interest also from the scientific point of view, as many compounds with the general formula CuxSy can be formed6 and each compound can exhibit some peculiarities, which could be possibly utilized in different applications. In nature, the most common ones are covellite, CuS and chalcocite, Cu2S.7There are various methods for the synthesis of copper sulfides, e.g. hydrothermal/solvothermal synthesis,8 hot injection method,9 thermolysis,10 microwave irradiation11 and electrodeposition.12 The ball milling method was also applied,13–18 as it offers all the advantages of the expanding field of mechanochemistry.19 These advantages include green, solvent-free synthesis of both organic20 and inorganic materials,21,22 the top-down synthesis of nanoparticles,23 the possibility to perform high temperature-demanding reactions under laboratory conditions or the possibility to mechanically activate the materials to make them suitable for specific applications.24,25
The precursors for the mechanochemical synthesis of CuxSy can be pure elements,13–16,26 or various copper- and sulfur-containing compounds.17,18 The reaction between copper and sulfur belongs among mechanically induced self-propagating reactions (MSR),27 which opens up a new field for the scientific investigation regarding temperature and pressure changes during the synthesis and their relation to the milling procedure.
Within this study, the mechanochemical synthesis of CuS and Cu2S from elemental precursors was pursued. The novelty of the present paper lies in the very rapid progress of the reaction, the detailed monitoring of the pressure changes during milling and the calculation of the contact temperature. The influence of the morphology of precursors, connected with their preparation way, was also investigated in detail.
2. Materials and methods
Different types of copper (Cu(M) – electrolytic from Merck, Germany; Cu(B) – Johnson Matthey Process Technologies, United Kingdom; Cu(Pa) – atomized powder from Pometon, Italy and Cu(Pe) – electrolytic from Pometon, Italy) and sulfur (S(C) – CG-Chemikalien, GmbH, Germany and S(B) – Johnson Matthey Process Technologies, United Kingdom) were used as chemicals without further purification.The milling process was realized in a planetary ball mill Pulverisette 7 Premium line (Fritsch, Germany) in a special tungsten carbide milling chamber with the volume 80 mL equipped with a sensor for the monitoring gas and temperature changes during milling – EASY GTM system (Fritsch, Germany). The mixtures of Cu and S in the stoichiometric ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were milled, with the aim to synthesize CuS and Cu2S, respectively. The milling conditions were as follows: sample mass – 3 g (1.9939 g of Cu and 1.0061 g of S in the case of Cu
1 were milled, with the aim to synthesize CuS and Cu2S, respectively. The milling conditions were as follows: sample mass – 3 g (1.9939 g of Cu and 1.0061 g of S in the case of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S ratio 1
S ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 2.3956 g of Cu and 0.6044 g of S in the case of Cu
1, and 2.3956 g of Cu and 0.6044 g of S in the case of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S ratio 2
S ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), atmosphere – air, 18 tungsten carbide milling balls with the diameter 10 mm, ball-to-powder ratio 47, rotation speed of the planet carrier– 500 min−1, milling time – from 10 seconds to 5 minutes.
1), atmosphere – air, 18 tungsten carbide milling balls with the diameter 10 mm, ball-to-powder ratio 47, rotation speed of the planet carrier– 500 min−1, milling time – from 10 seconds to 5 minutes.
The XRD patterns were obtained using a D8 Advance diffractometer (Bruker, Germany) with CuKα (40 kV, 40 mA) radiation. All samples were scanned from 10° to 87° with steps 0.03° and 6 s counting time. The crystallite size was estimated according to the integral breadth method, using the Diffracplus Topas software.
For the elemental sulfur determination, two grams of sample were placed inside a thick filter paper thimble, which was loaded into the main chamber of the Soxhlet extractor. The Soxhlet extractor was placed onto a flask containing 50 mL of extraction solvent (CS2, Acros Organics, Belgium) and a condenser was installed. The solvent was heated to reflux. When the Soxhlet chamber was almost full, the chamber was emptied, as the solvent ran back down to the distillation flask. This cycle was repeated three times. After extraction, the solvent was removed by a rotary evaporator, yielding the extracted compound. After weighting the extracted sulfur, the percentage of the non-reacted elemental sulfur in the sample was calculated according to the equation:
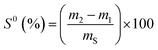 | (1) |
Scanning electron microscopy images of the samples were recorded by the utilization of MIRA3 FE-SEM microscope (TESCAN, Czech Republic) equipped with EDX detector (Oxford Instrument, United Kingdom).
3. Results and discussion
3.1. Influence of different precursors
We have used four different types of copper and two different types of sulfur within our experiments. They were characterized in terms of morphology, specific surface area and particle size distribution. The results are presented in the ESI (Fig. S1–S5 and Table S1†). It was found that the electrolytic types of copper possess a needle-like morphology (Fig. S1, S2a and S2b†), higher specific surface area values (Table S1†) and smaller grain size (Fig. S5†). On the other hand, the copper prepared in a different manner exhibited spherical morphology, lower specific surface area and larger grain size, thus suggesting the higher reactivity of the first group. Regarding sulfur, the morphology of the two types used was not significantly different and its structure can be easily distorted and should not be very significant during the milling process. It will be shown later that the character of copper used has a very significant impact on the reaction progress and that the used sulfur does not influence the reaction pathway, in terms of whether it is explosive or not. The role of different types of copper and sulfur was also investigated by Blachnik, et al.,14 where it was concluded that the pretreatment of copper, but not sulfur, significantly influences the reaction, which is in accordance with our results, as will be shown later. In order to show the impact of different copper and sulfur precursors, the results obtained for the electrolytic (Cu(M)) and atomized powder copper (Cu(Pa)) and for the two types of sulphur S(B and C) are presented in the paper. The rest of the results obtained for the combinations of all precursors used can be found in the ESI.†3.2. Gas pressure and temperature changes
The mechanochemical synthesis of the studied system led to the explosion, when electrolytic copper was used. In the case when the other copper was used, the explosion was not observed. This can be verified using the GTM system, by monitoring the gas pressure and temperature changes in the chamber during the milling. The changes were monitored for the Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S 1
S 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 1) and 2
1 (Fig. 1) and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures (Fig. 2).
1 mixtures (Fig. 2).
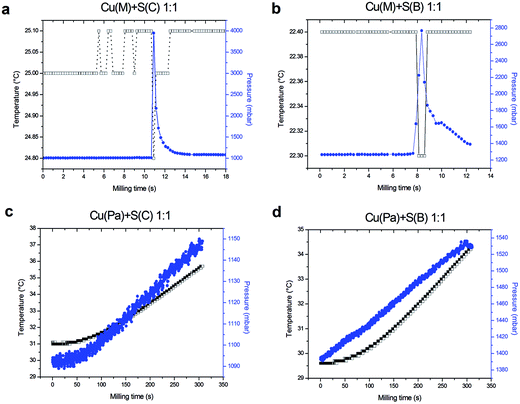
Fig. 1 documents completely different behavior in the case of electrolytic (a and b) and atomized powder (c and d) copper. In the former case, it can be seen that after the first few seconds of milling, the pressure did not change. After the activation time of approximately 10 seconds passed, a sharp increase in the pressure value was observed, documenting the explosion in the milling vessel and the ignition of the reaction. These are just selected experiments and the time varied by approximately ±5 seconds when the experiments were repeated. However, in all performed experiments, the explosion took place always within the first 15 seconds, when the electrolytic copper was used. The situation with the temperature was surprising, as the decrease at the moment of explosion was repeatedly observed (although only by 0.1 or 0.2 °C). This is very interesting phenomenon taking place every time, when the explosion documented by the dramatic increase of the pressure was observed, however we are unable to provide any scientific explanation for this process until now, as it might be also an artefact of the technical character. It might be connected with the sublimation of sulfur into a gaseous phase during the reaction. It is an endothermic process and could possibly take place in the mechanochemical reactions.28
When the atomized powder copper was used (Fig. 1c and d), a constant increase of the temperature and the pressure can be observed during the whole milling process lasting 5 minutes. The higher starting temperature is only the result of the fact that the milling chamber was not cooled down to the laboratory temperature prior to the experiments. No sharp increase of the pressure was observed in these experiments.
The type of sulfur does not seem to have a significant influence on the explosivity of the reaction (Fig. 1). Despite changing the character of sulfur, the explosivity of the reaction was dependent on the copper used.
The same experiments were performed with the Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S 2
S 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture (Fig. 2).
1 mixture (Fig. 2).
The same behavior, regarding the use of electrolytic (a) and atomized powder copper (b) was observed for the Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S 2
S 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
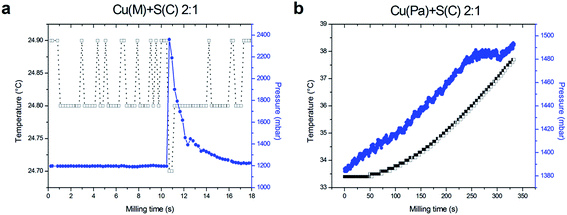
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture. In the first case, the explosive manner was observed, whereas in the latter, the constant increase of both measured characteristics was observed. In the case of the pressure increase in the latter case (Fig. 2b), it can be seen that at the time around 250 s, the pressure increase stops and it stands more-or-less on the constant level. This may be associated with the formation of small amount of the product, however, it has to be investigated in detail.
1 mixture. In the first case, the explosive manner was observed, whereas in the latter, the constant increase of both measured characteristics was observed. In the case of the pressure increase in the latter case (Fig. 2b), it can be seen that at the time around 250 s, the pressure increase stops and it stands more-or-less on the constant level. This may be associated with the formation of small amount of the product, however, it has to be investigated in detail.
If the two investigated mixtures are compared, it can be concluded that the stoichiometry does not influence the time of explosion. When comparing the shape of the pressure peaks, accompanying the formation of the products in both systems, we observe that in the case of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S 1
S 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture, Fig. 1a, the peak is the most intense, very narrow and fast decreasing, while for 2
1 mixture, Fig. 1a, the peak is the most intense, very narrow and fast decreasing, while for 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture (Fig. 2a) is the less intense, the widest, and slowly decreasing. This character of the pressure peaks may foreshadow some qualitative information about the crystallinity of the product, as will be shown later. The difference in the intensity and the shape of the pressure curve between the reactions can be partially related to the volatility of the sulfur. More sulfur results in the formation of more gas and therefore higher pressure is evidenced for the former mixture.
1 mixture (Fig. 2a) is the less intense, the widest, and slowly decreasing. This character of the pressure peaks may foreshadow some qualitative information about the crystallinity of the product, as will be shown later. The difference in the intensity and the shape of the pressure curve between the reactions can be partially related to the volatility of the sulfur. More sulfur results in the formation of more gas and therefore higher pressure is evidenced for the former mixture.
3.3. Ultrafast progress of the reaction and the phase composition
If the explosion was evidenced via pressure peaking, the milling was stopped immediately and the products were analyzed by XRD. Otherwise, the mixtures after the milling of 5 minutes were investigated. The results are presented in Fig. 3 and 4 for Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S 1
S 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures, respectively.
1 mixtures, respectively.
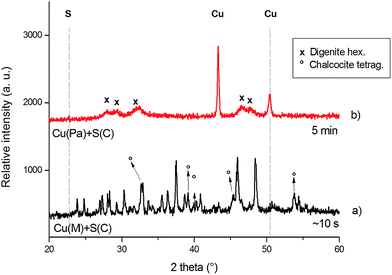 | ||
Fig. 4 XRD patterns of the milled Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S 2 S 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures. Time of milling is given in figure. The non-marked peaks in the pattern (a) belong to the monoclinic chalcocite. 1 mixtures. Time of milling is given in figure. The non-marked peaks in the pattern (a) belong to the monoclinic chalcocite. | ||
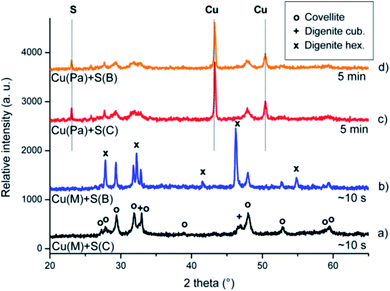
The XRD patterns document the ultrafast progress of the reaction in the case when the electrolytic copper (Cu(M)) was used. In the diffraction patterns of the products, formed by the explosive reaction, taken immediately after the explosion, the peaks corresponding to the elemental reactants have almost completely disappeared after a few seconds of reaction, contrary to other studies,13–15,26 in which the reaction was completed until tens of minutes.
When the XRD patterns of the product obtained from electrolytic copper, but different types of sulfur are compared (Fig. 3a and b), some difference can be observed. Although the peaks corresponding to the reactants have completely disappeared in both, it seems that the type of sulfur also influences the reaction pathway: while in the case of sulfur from CG – Chemikalien (S(C), Fig. 3a) the desired covellite was formed (68%) with a minor amount of cubic digenite, Cu1.8S (32%), using sulfur from the Johnson Matthey Process Technologies company (S(B), Fig. 3b) led to the formation of covellite (27%) but with major amount of hexagonal digenite (73%).
Rietveld analysis revealed the mean crystallite size of the main phase to be ∼15 nm in the former case, while larger crystallites of ∼42 nm are formed in the latter. Since the desired product (CuS) in this latter case is just minor, it was rejected from further considerations.
After the detailed examination of the XRD patterns of the post-explosion products of the reaction between Cu(M) and S(C) (Fig. 3a and 4a), it can be concluded that the desired sulfides have been prepared as major phases, namely hexagonal covellite, CuS (ICDD-PDF2 65-3561) and monoclinic chalcocite, Cu2S (ICDD-PDF2 033-0490). Minor phases have been also identified in both systems: in the synthesis of CuS, in addition to covellite also the cubic digenite (ICDD-PDF2 076-6653) and in the synthesis of Cu2S, in addition to the monoclinic chalcocite (86%) also the tetragonal phase (14%) (ICDD-PDF2 072-1071) were found. The mean crystallite size of the monoclinic chalcocite was found to be ∼65 nm. It can be claimed that the mean crystallite size corresponds well with the shape of the pressure peak during the explosion, namely, the larger and narrower peak shape, the finer crystallinity of the product.
The very fast progress of the reaction is not so surprising, as it can proceed even by mechanical mixing of both precursors in achate mortar, as was reported by Kristl, et al.29 Their finding further indicates that this reaction requires very low activation energy. However, in other works, no reaction was observed upon simple mixing.3,26 This hints to the fact, that when the air atmosphere and the copper prepared in the proper manner are applied, it can result in the ultrafast explosive reaction pathway. There is a possibility that in the first stage of the reaction, sulfur, being highly volatile, is transferred into gas phase and then reacts explosively with the solid copper.28
The product obtained without explosion, i.e. when the atomized powder copper was used, even after 5 minutes of milling contains a remarkable amount of unreacted copper, and also the main peaks of sulfur can be observed in the XRD patterns (Fig. 3c and d and 4b). However, it should be noted that in the case of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S 2
S 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture the traces of sulfur are only scarcely visible, this suggests the more rapid formation of Cu2S than CuS, which is in a good agreement with the thermodynamics of both sulfides (ΔG0298 = −20.8 kcal mol−1 for Cu2S and −12.87 kcal mol−1 for CuS).7 This is in contradiction with,14 where the formation of CuS was observed prior to Cu2S.
1 mixture the traces of sulfur are only scarcely visible, this suggests the more rapid formation of Cu2S than CuS, which is in a good agreement with the thermodynamics of both sulfides (ΔG0298 = −20.8 kcal mol−1 for Cu2S and −12.87 kcal mol−1 for CuS).7 This is in contradiction with,14 where the formation of CuS was observed prior to Cu2S.
In the ESI,† further XRD patterns describe the successful, very fast synthesis of CuS using the other electrolytic copper (Cu(Pe)), and a much slower progress, when different type of copper (Cu(B)) was used (Fig. S6†). This further confirms our observation, that the ultrafast explosive progress of the reaction is dependent on the copper used.
3.4. Elemental sulfur determination
As the detection limit of the XRD is not satisfactory to claim that the reaction is complete, the possible completion of the reaction was also monitored by the elemental sulfur determination conducted in a Soxhlet extractor. After weighing the extracted sulfur, the percentage of sulfur in the investigated samples, being the measure of the reaction progress, was calculated with the conclusion that the reaction is completed on 98% and 99% for Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S 1
S 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures, respectively. These results confirm the ultrafast consumption of elemental sulfur, S0 during the mechanochemical reaction with copper.
1 mixtures, respectively. These results confirm the ultrafast consumption of elemental sulfur, S0 during the mechanochemical reaction with copper.
3.5. Morphology of the products
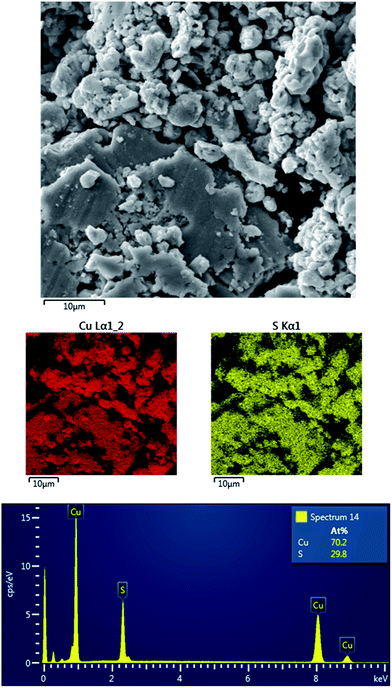
The morphology of the prepared copper sulfides was investigated by scanning electron microscopy. The micrographs are presented in Fig. 5.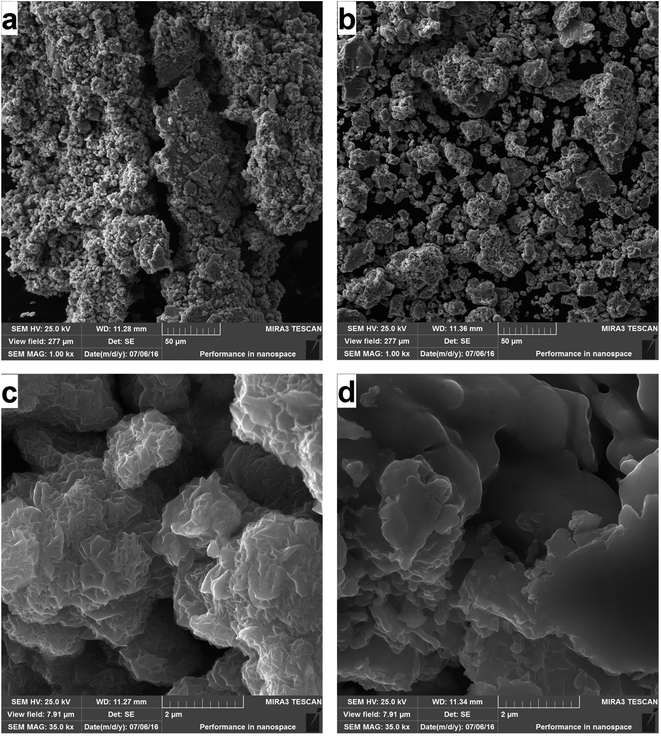 | ||
| Fig. 5 SEM micrographs of the prepared products: CuS (a) and Cu2S (b); highly magnified micrographs for CuS (c) and Cu2S (d). | ||
It is clearly seen that the morphology of the samples resembles the shapes of the burnt powder, as big agglomerates after such a short time of milling were observed. This is further proof of the explosive reaction pathway. The prepared CuS seems to be more agglomerated than Cu2S, as the latter contains smaller grains (Fig. 5a and b). In order to investigate the morphology of the individual grains and potentially, the presence of nanoparticles, highly magnified micrographs were obtained (Fig. 5c and d). The difference in the morphology can be observed also at this level. Whereas the morphology of CuS seems to be more rugged, the prepared Cu2S exhibits more smooth surfaces. The micrographs of both structures suggest the presence of the nanoparticles in the agglomerates, however in the case of Cu2S, the nanoparticles should be of larger size, which corresponds with the results obtained from the Rietveld analysis. As the powders were prepared by high-energy milling, the nanoparticles are not present as individual, but are highly agglomerated. This is a common phenomenon in mechanochemistry.23,30
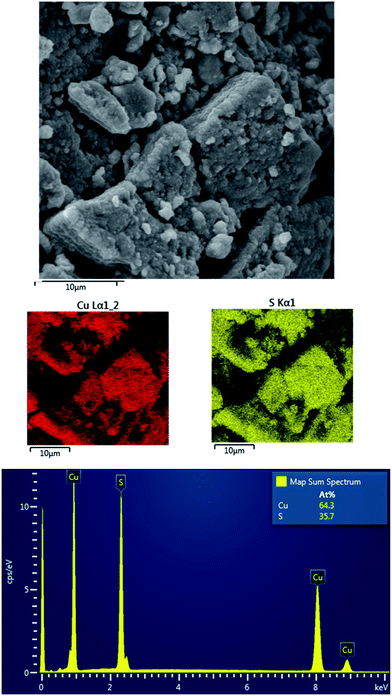
The chemical composition of the products was investigated by the EDX and the elemental mapping of both products was performed. The results for CuS and Cu2S are provided in Fig. 6 and 7, respectively.
The results show the homogeneous distribution of both elements, which confirms the complete consumption of the reactants, as in the other case, differences in the distribution would be observed. Regarding the results of the EDX analysis, only copper and sulfur elements were evidenced, so no oxidation products were formed, despite milling on air. The Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S atomic ratio in the case of CuS (Fig. 6) is not 50
S atomic ratio in the case of CuS (Fig. 6) is not 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, in accordance with the interpretation of the XRD patterns, where also digenite, Cu1.8S was detected. In the case of Cu2S, the Cu
50, in accordance with the interpretation of the XRD patterns, where also digenite, Cu1.8S was detected. In the case of Cu2S, the Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S atomic weight ratio was 70
S atomic weight ratio was 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, which is higher than should be for the pure Cu2S, however the error is within the measurement range of the method. Moreover, the fact that the EDX should be used on the flat surfaces under the defined angle should be also taken into account, so the results should be not completely accurate for the non-homogeneous surfaces of the milled samples.
30, which is higher than should be for the pure Cu2S, however the error is within the measurement range of the method. Moreover, the fact that the EDX should be used on the flat surfaces under the defined angle should be also taken into account, so the results should be not completely accurate for the non-homogeneous surfaces of the milled samples.
3.6. Calculation of contact temperature
Following the paper by Urakaev,31 it is possible to calculate the contact temperature at the time of explosion for both systems. This temperature can be calculated both from the experimental data provided by the GTM system (temperature and pressure changes during milling) or from the theoretical thermodynamic parameters. In our case, the temperature increase in the mill, ΔT, for the formation of CuS is 950 °C whereas for Cu2S, it is only 700 °C. The theoretical estimation of the adiabatic temperature in the milling vessel according to the complete reaction of the copper sulfides formation should be 980 °C for CuS and 1080 °C for Cu2S. The calculations show that there is a satisfactory agreement between the temperature increases calculated by both approaches for the CuS system, whereas for Cu2S, the results do not match. This could possibly be a result of the complexity of the Cu2S system with different co-existing crystallographic structures.4. Conclusions
The mechanochemical synthesis of covellite, CuS and chalcocite, Cu2S within few seconds was successfully realized. The explosive character of the solid-state reaction between copper and sulfur was confirmed by observing a significant sharp increase in the pressure inside the milling chamber after few seconds. Upon that, the reaction was almost completed. The reaction pathway is strongly influenced by the morphology of the copper, concretely, the electrolytically prepared one favors the ultrafast reaction pathway. The morphology of the products resembles burnt powder after the explosion and the elemental mapping and the EDX analysis confirm, in majority, the formation of the desired products. The temperatures at the contact sites, calculated from the experimental data, were 950 °C and 700 °C for CuS and Cu2S, respectively. The ultrafast synthesized products, upon slight purification, can be potentially used for multidisciplinary applications.Acknowledgements
This work was supported by the by the Slovak Research and Development Agency under the contract No. APVV-14-0103. The support of other organizations, namely German Federal Ministry of Education and Research (project IB-COMSTRUC-010), Slovak Grant Agency VEGA (project 2/0027/14), European Regional Development Fund (project nanoCEXmat II, ITMS 26220120035), Republic of Kazakhstan (the contract number 0130/PTsF-14) and RFBR grant 15-05-03980A of Russia is also gratefully acknowledged.References
- P. Roy and S. K. Srivastava, CrystEngComm, 2015, 17, 7801–7815 RSC.
- A. Ghosh and A. Mondal, Appl. Surf. Sci., 2015, 328, 63–70 CrossRef CAS.
- K. Ramasamy, M. A. Malik, N. Revaprasadu and P. O'Brien, Chem. Mater., 2013, 25, 3551–3569 CrossRef CAS.
- Y. K. Hsu, Y. C. Chen and Y. G. Lin, Electrochim. Acta, 2014, 139, 401–407 CrossRef CAS.
- S. Goel, F. Chen and W. B. Cai, Small, 2014, 10, 631–645 CrossRef CAS PubMed.
- P. Lukashev, W. R. L. Lambrecht, T. Kotani and M. van Schilfgaarde, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 195202 CrossRef.
- D. J. Vaughan and J. R. Craig, Mineral Chemistry of Metal Sulfides, Cambridge University Press, Cambridge, 1978 Search PubMed.
- S. K. Goswami, J. Kim, K. Hong, E. Oh, Y. M. Yang and D. Yu, Mater. Lett., 2014, 133, 132–134 CrossRef CAS.
- P. L. Saldanha, R. Brescia, M. Prato, H. B. Li, M. Povia, L. Manna and V. Lesnyak, Chem. Mater., 2014, 26, 1442–1449 CrossRef CAS.
- L. Chen, Y. B. Chen and L. M. Wu, J. Am. Chem. Soc., 2004, 126, 16334–16335 CrossRef CAS PubMed.
- M. D. Xin, K. W. Li and H. Wang, Appl. Surf. Sci., 2009, 256, 1436–1442 CrossRef CAS.
- P. Kar, S. Farsinezhad, X. J. Zhang and K. Shankar, Nanoscale, 2014, 6, 14305–14318 RSC.
- K. Wang and G. L. Tan, Curr. Nanosci., 2010, 6, 163–168 CrossRef CAS.
- R. Blachnik and A. Muller, Thermochim. Acta, 2000, 361, 31–52 CrossRef CAS.
- T. Ohtani, M. Motoki, K. Koh and K. Ohshima, Mater. Res. Bull., 1995, 30, 1495–1504 CrossRef CAS.
- Z. Y. Ou and J. H. Li, RSC Adv., 2014, 4, 51970–51976 RSC.
- E. Godočíková, P. Baláž, J. M. Criado, C. Real and E. Gock, Thermochim. Acta, 2006, 440, 19–22 CrossRef.
- X. B. Wang, C. Q. Xu and Z. C. Zhang, Mater. Lett., 2006, 60, 345–348 CrossRef CAS.
- W. Jones and M. D. Eddleston, Faraday Discuss., 2014, 170, 9–34 RSC.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC.
- J. Qu, Q. W. Zhang, X. W. Li, X. M. He and S. X. Song, Appl. Clay Sci., 2016, 119, 185–192 CrossRef CAS.
- V. Šepelák, A. Düvel, M. Wilkening, K. D. Becker and P. Heitjans, Chem. Soc. Rev., 2013, 42, 7507–7520 RSC.
- P. Baláž, M. Achimovičová, M. Baláž, P. Billik, Z. Cherkezova-Zheleva, J. M. Criado, F. Delogu, E. Dutková, E. Gaffet, F. J. Gotor, R. Kumar, I. Mitov, T. Rojac, M. Senna, A. Streletskii and K. Wieczorek-Ciurowa, Chem. Soc. Rev., 2013, 42, 7571–7637 RSC.
- Z. Ou, J. Li and Z. Wang, Environ. Sci.: Processes Impacts, 2015, 17, 1522–1530 CAS.
- G. Cagnetta, J. Robertson, J. Huang, K. L. Zhang and G. Yu, J. Hazard. Mater., 2016, 313, 85–102 CrossRef CAS PubMed.
- S. Li, Z. H. Ge, B. P. Zhang, Y. Yao, H. C. Wang, J. Yang, Y. Li, C. Gao and Y. H. Lin, Appl. Surf. Sci., 2016, 384, 272–278 CrossRef CAS.
- L. Takacs, Prog. Mater. Sci., 2002, 47, 355–414 CrossRef CAS.
- F. K. Urakaev, A. I. Bulavchenko, B. M. Uralbekov, I. A. Massalimov, B. B. Tatykayev, A. K. Bolatov, D. N. Dzharlykasimova and M. M. Burkitbayev, Colloid J., 2016, 78, 210–219 CrossRef CAS.
- M. Kristl, I. Ban and S. Gyergyek, Mater. Manuf. Processes, 2013, 28, 1009–1013 CAS.
- P. Baláž, Mechanochemistry in Nanoscience and Minerals Engineering, Springer, Berlin Heidelberg, 2008 Search PubMed.
- F. K. Urakaev, Combust. Sci. Technol., 2013, 185, 723–734 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra20588g |
| This journal is © The Royal Society of Chemistry 2016 |