Biomimetic thermoplastic polyurethane porous membrane with hierarchical structure accelerates wound healing by enhancing granulation tissue formation and angiogenesis
Qiang Lei†
a,
Zhichao Li†b,
Rui Xua,
Yuzhen Wanga,
Haisheng Lia,
Ying Wanga,
Menglong Liua,
Sisi Yanga,
Rixing Zhana,
Jian Zhaob,
Bo Liub,
Xiaohong Hua,
Xiaorong Zhanga,
Weifeng Hea,
Jun Wua,
Hesheng Xia*b and
Gaoxing Luo*a
aInstitute of Burn Research, State Key Laboratory of Trauma, Burn and Combined Injury, Southwest Hospital, the Third Military Medical University, Chongqing, 400038, China. E-mail: logxw@hotmail.com; Fax: +86-023-65461677; Tel: +86-023-68754173
bState Key Laboratory of Polymer Materials Engineering, Polymer Research Institute of Sichuan University, Chengdu, 610065, China. E-mail: xiahs@scu.edu.cn; Fax: +86-028-85460535; Tel: +86-028-85460535
First published on 12th October 2016
Abstract
Thermoplastic polyurethane (TPU) is an appropriate material for wound dressings, and has been processed into a variety of forms to match the structural and morphological features required by current clinical demands. The aim of the present work was to manufacture biomimetic porous membranes composed of TPU and investigate the effects on wound healing. The hierarchical thermoplastic polyurethane porous membrane (HTPM) were prepared using a novel, simple and tunable method that combines immersion precipitation with particle leaching. Physical testing indicated that the HTPM possess is more favorable mechanical properties than conventional products, and the inner membrane structure is more similar to that of natural skin. Meanwhile, the HTPM exerted no adverse effects on fibroblast viability and proliferation by a Cell Counting Kit-8 assay. Furthermore, the histological and Western blot results indicated that wound re-epithelialization, granulation tissue formation and angiogenesis were enhanced when skin defects were covered with the HTPM, which significantly accelerated wound healing. These results demonstrated that the bilayer HTPM have therapeutic promise as wound dressings.
1 Introduction
The skin is an indispensable component of the human body that forms an effective barrier between the organism and the environment to prevent the invasion of pathogenic microorganisms, resist chemical and physical assaults, and reduce the excessive loss of body fluid and electrolytes.1 As such, once the integrity of the skin disrupted, especially in the form of extensive skin defects, the material should be applied immediately to cover the wound.2–4 The gold standard in clinic such injuries treatment is autologous skin grafting,5 but healthy donor sites are usually extremely limited in those conditions. Allografting is considered a potential alternative;6,7 however, its implementation depends on skin bank availability, and there are risks associated with infectious viral diseases. Herein, wound dressings used as temporary coverings play a positive role in treating extensive skin defects.8,9 Ideally, such wound dressings have antimicrobial activity, retain moisture, are easily removable, can be changed without pain and are available in different sizes.10,11The structure of wound dressings has gained attention in an effort to manufacture products that exhibit several required functions. For example, Xu et al.12 reported that a bilayer silicone rubber membrane with a specific microporosity enhanced wound re-epithelialization and contraction. Additionally, Wang et al.13 produced artificial dermis with a “sandwich” structure designed with a gradient of pore sizes that promoted wound healing. Although huge efforts have been made to manufacture an ideal wound dressing, there are still some problems with currently available products due to their individual characteristics and disadvantages, such as poor physical properties, complicated fabricate processes and immune reaction.14–16
In a previous study, we generated collagen/elastin-based three-dimensional images of human dermis.17 Based on this work and inspired by bionics, we have developed a novel method for preparing a biomimetic porous membrane composed of thermoplastic polyurethane (TPU). The membrane fabrication procedure not only was relatively simple compared with conventional techniques but also enabled the porosity, water vapor transmission rate (WVTR), and thickness of the membrane to be tuned.18 Additionally, the biomimetic TPU porous membrane was manufactured according to the morphological features of natural skin determined in previous research.17
In this study, hierarchical TPU porous membrane (HTPM) with a bilayer structure were prepared and tested. In addition to exhibiting superior mechanical properties, suitable porosity and satisfactory WVTR compared with conventional TPU porous membrane (CTPM), the Cell Counting Kit (CCK)-8 assay confirmed that the HTPM had no adverse effects on fibroblast viability and proliferation. Moreover, the HTPM could temporarily acts as a mechanical barrier for wound sites and supply a suitably moist microenvironment for tissue repair, thereby enhancing re-epithelialization, granulation tissue formation and angiogenesis, which obviously accelerated wound healing. These results revealed that the bilayer HTPM have promising as dressings for wound protection or wound bed preparation.
2 Materials and methods
2.1 Materials and animals
BALB/c mice (approximately 30 g, male) were purchased from the Experimental Animal Department of the Third Military Medical University (TMMU). The animals were raised for 1 week individually before the experiments, with free access to water and autoclaved rodent chow under standardized conditions (i.e., relative humidity: 50%; circadian rhythm: 12 h; room temperature: 25 °C).Fibroblasts were obtained from the foreskin of 6 to 12 year-old boys, with their guardian's permission and written consent.
In this study, all animal protocols were permitted by the Institutional Animal Care and Use Committee of the Third Military Medical University (TMMU) Chongqing China, and all animal experiments followed the Regulation on the Management of Laboratory Animals, which was issued by Chinese Association for Laboratory Animal Sciences (CALAS). Meanwhile, the application of human foreskin sample was approved by the Ethics Committee of the Southwest Hospital (no. 20140926).
2.2 Preparation of HTPM and conventional TPU porous membranes (CTPM)
The combined process of immersion precipitation and particle leaching was used to prepare the HTPM. In brief, 25 g of TPU granules, 200 mL of N,N-dimethyl formamide (DMF) and a certain amount of Na-citrate powder were combined to form a homogeneous DMF/TPU/Na-citrate mixture. After deaeration under reduced pressure, air bubbles evolve to entrapped, the DMF/TPU/Na-citrate solution was cast in a polytetrafluoro-ethylene (PTFE) mold. The PTFE mold was then immersed in ethanol. After TPU solidification, the mold was removed and the membrane was immersed in deionized water to extract the Na-citrate particles. Finally, HTPM was obtained after being dried at 40 °C for 6 h.CTPM was prepared by using the particle leaching process alone. The DMF/TPU/Na-citrate solution was cast in a PTFE mold and then baked at 90 °C to extract the solvent. The membrane was directly immersed in deionized water to remove the Na-citrate. Finally, the membrane was dried at 40 °C for 6 h.
2.3 HTPM structural observations and mechanical property measurements
The pore structure of the HTPM was observed by scanning electron microscopy (SEM, Inspect F, Philips, Netherlands). The HTPM specimens were frozen in liquid nitrogen and fractured. The upper, lower and fractured surfaces were coated with gold to prevent surface charging. SEM observations were operated at a voltage of 20 kV.The mechanical properties of the membranes were measured by tensile testing. In brief, the membrane specimens were cut into a dumb-bell shape and tested by using an Instron 5567 materials testing system (Instron, USA). These specimens were stretched to failure at a velocity of 50 mm min−1. A total of 5 specimens were tested in each group.
2.4 Determination of the HTPM WVTR
To determine the moisture permeability of the HTPM, the WVTR was measured according to the American Society for Testing and Materials standard protocol.12 Specimens were cut into discs with an area of 33 mm2 and examined using a WVTR testing system (W3/030, Labthink, China). The incubator was set to 37.5 °C and 90% relative humidity. All measurements were repeated 5 times.2.5 Primary fibroblast cultures
Fibroblasts were isolated from normal foreskin, as described by Smith et al.19 First, penile prepuce samples were harvested and sterilized with iodophor; then, the samples were washed with PBS and immersed in 0.5 mg mL−1 Dispase II (Roche, Cat 04942078001) at 4 °C for 12 h to enable the careful removal of the epidermis layer. The dermal tissue was cut into small pieces approximately 10 mm × 10 mm in size, immersed in 3 mL of 0.5% trypsin (Boster, China) at 37 °C, and then digested for 8 min. Subsequently, 10 mL of Dulbecco's Modified Eagle's Medium (DMEM, Gibco, USA) with 10% fetal bovine serum (Gibco, USA) was added to neutralize the trypsin. After the samples were centrifuged (1000 rpm, 6 min), the supernatant was discarded, and the pellet was resuspended with DMEM containing 10% fetal bovine serum, streptomycin (100 U mL−1) and penicillin (100 U mL−1), followed by incubation in a 5% CO2 incubator at 37 °C.2.6 The CCK-8 assay
The third-generation fibroblasts were seeded in 96-well plates at 3 × 103/100 μL per well. Then, the culture medium was replaced with the leach liquor extracting from HTPM and CTPM, and untreated medium was used as a blank control. The biocompatibility of the materials was assessed using CCK-8 (Dojindo, Kyushu, Japan) proliferation assays on days 1, 3, 5 and 7 after seeding. In brief, at each time point, the medium was replaced with DMEM, and 10 μL of CCK-8 solution was added to each well, followed by incubation at 37 °C for 2 h. The mean optical density (OD) value was determined at 450 nm using an enzyme-linked immunosorbent assay reader (Thermo Varioskan Flash, USA),20 and the cell viability (%) was expressed in percentage relative to a control group.212.7 Effect of coverage with the prepared TPU membranes on the healing of murine full-thickness skin defect wound
The effect of using the porous TPU membranes to cover wounds on the healing process was evaluated using BALB/c mice and the dorsal skin defect model.22 The hair was first shaved, and then a skin defect was made using a circular puncher (ϕ = 4 mm). After that, the wounds were photographed to document the initial wound area and were then covered with different materials. The wound dressings were replaced and the wounds were photographed every other day. The control group consisted of wounds that did not receive any treatment, while the others were treated with HTPM, CTPM or Vaseline gauze. Photoshop (CS5, Adobe, USA) was used to carefully trace the margins of each wound, and calculate the number of pixels encompassed by each wound tracing. The number of pixels was then converted into square millimeters. The extent of wound healing was calculated using the following formula:| W= (I − An)/I × 100% |
2.8 Histological observations
Mice were sacrificed on days 3 and 7 post-wounding, and full-thickness wound tissues were harvested, fixed in paraformaldehyde at 4% (v/v), embedded in paraffin and sliced at a thickness of 5 μm. The sections were stained with hematoxylin and eosin (H&E) for histological analysis. The granulation thickness and length of new epithelium were determined by two independent researchers using Image J (1.50i, National Institute of Healthy, USA). The length of new epidermis was defined as the distance between the edge of the unwounded epidermis and the leading edge of the newly generated epidermis.24 The sections were imaged using an optical microscope (DM6000B, Leica, Germany).2.9 Western blot analysis to detect proliferating cell nuclear antigen (PCNA) and CD31 expression
To quantitatively assess cell proliferation and angiogenesis in the wound tissues of the different groups, the expression levels of two markers, PCNA and CD31, were determined by Western blot on day 5 post-wounding. In brief, full-thickness wound tissues approximately 10 mm × 10 mm in size, including newly regenerated epidermis and granulation tissue, were harvested and immediately put into liquid nitrogen. After these samples were ground, lysis buffer (KeyGen, China) was added. The samples were then rotated for 15 min at 4 °C, the homogenates were centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min, and then the supernatants were collected. Protein concentrations were determined via the bicinchoninic acid method in accordance with the instructions for the Varioskan Flash (Thermal Scientific, USA). Equal amounts of protein were boiled for 5 minutes before being loading onto 8% SDS-PAGE gels. Protein samples of 50 μg were used for the Western blot analysis. Electrophoresis was performed at 80 volts for 30 min and then at 100 volts for 90 min. The proteins were then transferred to PVDF membranes at 100 volts for 90 min. The PVDF membranes were blocked with tris-buffered saline (TBS) containing 5% bovine serum albumin (Biosharp, China) for 3 hours at 25 °C, and the membranes were then incubated with anti-PCNA antibody (ab15497, Abcam, UK) at a 1
000 rpm for 15 min, and then the supernatants were collected. Protein concentrations were determined via the bicinchoninic acid method in accordance with the instructions for the Varioskan Flash (Thermal Scientific, USA). Equal amounts of protein were boiled for 5 minutes before being loading onto 8% SDS-PAGE gels. Protein samples of 50 μg were used for the Western blot analysis. Electrophoresis was performed at 80 volts for 30 min and then at 100 volts for 90 min. The proteins were then transferred to PVDF membranes at 100 volts for 90 min. The PVDF membranes were blocked with tris-buffered saline (TBS) containing 5% bovine serum albumin (Biosharp, China) for 3 hours at 25 °C, and the membranes were then incubated with anti-PCNA antibody (ab15497, Abcam, UK) at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 dilution, anti-CD31 antibody (ab28364, Abcam, UK) at a 1
1000 dilution, anti-CD31 antibody (ab28364, Abcam, UK) at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 dilution, and anti-tubulin antibody (Sungene, China) at a 1
1000 dilution, and anti-tubulin antibody (Sungene, China) at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 dilution at 4 °C overnight. The membranes were subsequently washed with TBS containing 1% Tween-20 (TBST) 5 times and then incubated with HRP-labeled goat anti-rabbit secondary antibody (Zhongshan Biology Company, China) at a 1
2000 dilution at 4 °C overnight. The membranes were subsequently washed with TBS containing 1% Tween-20 (TBST) 5 times and then incubated with HRP-labeled goat anti-rabbit secondary antibody (Zhongshan Biology Company, China) at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 dilution for 1 h at 25 °C. The PDVF membranes were washed with TBST 5 times and then visualized using chemiluminescence (Thermal Scientific, USA).25
2000 dilution for 1 h at 25 °C. The PDVF membranes were washed with TBST 5 times and then visualized using chemiluminescence (Thermal Scientific, USA).25
2.10 Statistical analysis
All data are presented as the mean ± standard deviation (SD). T-tests and one-way ANOVA were used to evaluate statistical significance, followed by post hoc least significant difference tests. Values of p < 0.05 were considered significant.3 Results
3.1 HTPM exhibited favorable mechanical properties and suitable WVTR
| Thickness (mm) | Pore sizes (μm) | Pore density (pores/μm2) | |
|---|---|---|---|
| Top surface | — | 2.11 ± 0.62 | (1.06 ± 0.37) × 10−2 |
| Upper layer | 1.16 ± 0.04 | 76.17 ± 24.63 | (2.40 ± 0.64) × 10−4 |
| Sublayer | 0.46 ± 0.05 | 108.63 ± 26.55 | (0.68 ± 0.26) × 10−4 |
| Bottom layer | — | 92.18 ± 18.36 | (0.74 ± 0.10) × 10−4 |
3.2 HTPM had no adverse effects on fibroblast viability and proliferation
Compared to the controls, the fibroblasts in the HTPM group displayed a normal morphology as observed by microscopy, and the number of fibroblasts exhibited an increasing trend. The CCK-8 assay revealed that the leach liquor extracting from the HTPM had no detrimental effects on fibroblast viability and proliferation. There were no differences in cell viability between the HTPM and CTPM groups on days 1, 3, 5, or 7 post-seeding, which demonstrated that the HTPM and CTPM both are suitably biocompatible (Table 2, n = 4 per group).| Day 1 | Day 3 | Day 5 | Day 7 | |
|---|---|---|---|---|
| CTPM | 90 ± 8 | 241 ± 11 | 416 ± 20 | 620 ± 32 |
| HTPM | 105 ± 11 | 247 ± 19 | 421 ± 19 | 679 ± 53 |
3.3 HTPM accelerated wound healing and shortened wound closure time compared with CTPM, Vaseline gauze and the control
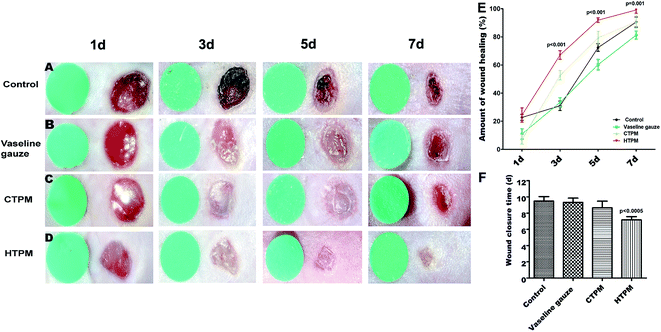
Images of the gross appearance of the wounds at the set time points are shown in Fig. 3A–D. We determined the extent of wound healing that occurred in the mouse wound healing model, and the data are shown in wound closure curves. On the 5th day, the average extent of wound healing in the control, Vaseline gauze, CTPM and HTPM groups was 60.3%, 72.4%, 79.4% and 91.9%, respectively. The results revealed that wound healing was significantly enhanced by the HTPM treatment, as the extent of wound healing was largest in the HTPM group (Fig. 3E, p < 0.001). The average wound closure time in the control, Vaseline gauze, CTPM and HTPM groups was 9.5 days, 9.3 days, 8.67 days and 7.17 days, respectively (Fig. 3F; HTPM vs. CTPM, p < 0.0005).3.4 The re-epithelialization and granulation tissue formation were enhanced when covered with HTPM
Images of the wound tissue at days 3 and 7 post-wounding are shown in Fig. 4A–I. The length of newly regenerated epidermis was significantly longer in the HTPM group than in the control, Vaseline gauze and CTPM groups at day 3 post-wounding (Fig. 4J, HTPM vs. control, p = 0.005; HTPM vs. CTPM, p < 0.05). However, no difference was found in the length of regenerated epidermis between the HTPM and CTPM groups at day 7 post-wounding, but that of the HTPM group was obviously longer than that of the control group (HTPM vs. control, p < 0.05).The thickness of the granulation tissue was histologically measured only at day 7 post-wounding because most wounds form bare granulation tissue and specimens are difficult to harvest. Images of the granulation tissue samples are shown in Fig. 5A–D. The granulation tissue thickness in the HTPM group was significantly larger than that in the other groups at day 7 post-wounding (Fig. 5E, HTPM vs. control, p = 0.001; HTPM vs. Vaseline gauze, p < 0.001; HTPM vs. CTPM, p < 0.05).
3.5 The HTPM enhanced cell proliferation and angiogenesis
The OD values of the PCNA band were highest in the HTPM group (Fig. 6B, HTPM vs. CTPM, p < 0.05; HTPM vs. control p = 0.001), indicating that cell proliferation was significantly promoted in the granulation tissue and newly regenerated epidermis compared with the control, Vaseline gauze and CTPM groups, which corresponded with the histological observations (Fig. 4). The OD values of the CD31 band were significantly higher in the HTPM group than in the other groups, which revealed active angiogenesis in the wound tissue (Fig. 6C, HTPM vs. CTPM, p = 0.01; HTPM vs. control p < 0.005).4 Discussion
During the past few decades, researchers have made great progress in understanding the mechanisms involved in wound healing,28,29 and various wound dressings have been produced to satisfy all types of clinical requirements, such as the ability to exhibit anti-bacterial and anti-fouling properties, release special factors, and demonstrate favourable absorption.30–32 Recently, more attention is being paid to the structural and morphological features of wound dressings in order to provide more suitable microenvironments for tissue repair. Biomimetic materials that mimic the structure of natural skin are considered promising wound dressings. However, these products are not widely used due to individual characteristics and drawbacks, such as high cost and time consumption, poor physical properties, and complicated fabrication processes.15,16Due to its favourable mechanical properties, oxygen permeability, chemical stability, economical efficiency, nontoxicity and biocompatibility, TPU has already been approved by the FDA and is widely used in medical products.33,34 By combining the immersion precipitation process with particle leaching, porous membranes composed of TPU with a hierarchical structure were manufactured in a novel and simple manner (Fig. 7). During the immersion precipitation step, the exchange of DMF and ethanol across the interface resulted in a TPU-rich phase and a TPU-poor phase. The solidification of TPU in ethanol was a bimodal phase separation (Fig. 7 phase diagram, a); the composition path remained in the metastable region for a sufficient time, allowing nuclei to form and grow into a globule-like TPU-poor phase. Phase separation was continued until the TPU-rich phase was solidified and the TPU-poor phase turned into spherical and discontinuous pores.35–38 Na-citrate particles precipitated during this liquid–liquid phase separation process. As a result, spherical and discontinuous pores formed in the upper layer (Fig. 8B), and most Na-citrate particles were deposited at the bottom. After removing the Na-citrate particles, irregular and continuous pores were formed in the lower layer (Fig. 8C). However, only irregular pores formed by extracting the pore-forming agent when preparing the CTPM. As a consequence, two different layers were present in a single membrane (Fig. 8A). Compared with the conventional products, the bilayer structure of the HTPMs was more similar to the natural structure of skin.
 | ||
| Fig. 7 The formation of different pore structures in the CPTM and HTPM and the ternary liquid–liquid phase diagram of polymer/solvent/nonsolvent. | ||
The thick upper layer, with an average pore size of 2.11 μm, which is smaller than most pathogens, could function as the epidermis (Fig. 1B). This layer could not only reduce water loss and provide a moist environment for wound repair but also act as an effective barrier between the tissue and the environment and prevent the invasion of pathogenic microorganisms. The inner structure was designed to have a sponge-like structure and a large pore size (Fig. 8), based on evidence showing that the pore diameter affects cell conformation and proliferation; a microporous hydrogel with a pore size of 80 to 120 μm has been reported to enhance chondrocyte proliferation.39 Meanwhile, the sponge-like structure provides a swelling capability suitable for absorbing excess exudate and maintaining a wound environment that is moist but not wet. As such, compared with the conventional products, the bilayer structure of the HTPM is more similar to that of natural in multiple ways (Table 1). In addition, the favourable mechanical properties and suitable WVTR (Fig. 2) of the HTPM indicate the potential of this material for application as a wound dressing.
In this study, the extract liquid of HTPM had no adverse impact on fibroblast viability and proliferation compared with the control group, demonstrating the biocompatibility of the HTPM (Table 2). Furthermore, the effect of the HTPM on wound healing was assessed, and the results showed that wound healing was significantly accelerated in the HTPM group compared to the other groups (Fig. 3), which was attributed to the more suitable, moist microenvironment provided by the HTPM (Fig. 2C). Wound re-epithelialization has been reported to occur more rapidly in a suitably moist environment than under dry conditions.40 In our study, on the 7th day post-wounding, nearly 99.1% wound closure was achieved in the HTPM-treated mice (Fig. 3D and E), which was significantly greater than that observed in the blank control, Vaseline gauze and CTPM groups. Moreover, the average wound closure time was 7.17 days (Fig. 3F) in the HTPM groups, which was significantly shorter than that in the other groups.
To further detect the mechanism by which the HTPM enhanced wound healing, the length of regenerated epidermis and the granulation tissue thickness were measured. Wound re-epithelialization usually begins within hours after an injury. In this study, the length of the epithelial tongue was significantly increased in the HTPM group at days 3 and 7 post-wounding compared to that in the other groups (Fig. 4), and the same result was observed for granulation tissue thickness at day 7 post-wounding (Fig. 5). Granulation tissue, which includes macrophages, fibroblasts, and blood vessels, contains important factors for wound healing. Angiogenesis is also a related process that supports granulation tissue formation and provides nutrition and oxygen necessary for tissue repair.41 The expression levels of the proliferation marker PCNA and the angiogenic marker CD31 were detected by Western blot at day 5 post-wounding, and the expression levels of both markers were significantly higher in the HTPM group than in the CTPM and control groups (Fig. 6). These results demonstrated that the HTPM significantly promoted cell proliferation and angiogenesis in the wound sites, which would obviously accelerate wound healing.
In summary, we optimized a simple and tunable method for preparing biomimetic porous membranes, HTPM, which exhibit favourable mechanical properties, are nontoxic and mimic the structure of natural human skin. Applied as a wound dressing, this material could protect cutaneous wounds and promote wound healing. The length of regenerated epidermis and the granulation tissue thickness were measured; moreover, the expression levels of PCNA and CD31 were detected. The results clearly showed that the HTPM accelerated the wound healing process. We speculate that the suitably moist microenvironment provided by the membrane and its biomimetic structure were the factors responsible for the observed effects on wound healing. However, our study has some limitations, e.g., the structure of natural human skin was not completely replicated. As a next step, we will optimize the HTPM structure and assess the potential influences of pore structure on wound healing.
5 Conclusions
We manufactured a new type of TPU porous membrane with favourable mechanical properties by combining immersion precipitation with particle leaching. The HTPM had a biomimetic structure and were designed with a gradient of pore sizes. These membranes could promote wound healing via favouring granulation tissue formation, wound re-epithelialization and angiogenesis; as such, this material could potentially be used as a wound dressing in the future.Conflict of interest
The authors declare that they have no conflict of interest.Acknowledgements
This research was supported by grants from China's NSFC grants program (81471870, 30957768, 30973116, and 81171809), the “863” project (2012AA020504) and the Key Project of Military Medical Plan (AWS11J012-05, BWS11J039).References
- E. Proksch, J. M. Brandner and J. Jensen, Exp. Dermatol., 2008, 17, 1063–1072 CrossRef PubMed.
- M. M. Soltan Dallal, R. Safdari, H. Emadi Koochak, S. Sharifi-Yazdi, M. R. Akhoondinasab, M. R. Pourmand, A. Hadayatpour and M. K. Sharifi-Yazdi, Burns, 2016, 42, 578–582 CrossRef CAS PubMed.
- A. P. Lesher, R. H. Curry, J. Evans, V. A. Smith, M. T. Fitzgerald, R. A. Cina, C. J. Streck and A. V. Hebra, J. Pediatr. Surg., 2011, 46, 1759–1763 CrossRef PubMed.
- L. W. Chan, C. H. Kim, X. Wang, S. H. Pun, N. J. White and T. H. Kim, Acta Biomater., 2016, 31, 178–185 CrossRef CAS PubMed.
- Z. Jan Ekovi, J. Plast. Reconstr. Aesthetic Surg., 2008, 61, 240–244 CrossRef PubMed.
- J. R. Saffle, Clin. Plast. Surg., 2009, 36, 627–641 CrossRef PubMed.
- E. Moerman, E. Middelkoop, D. Mackie and F. Groenevelt, Burns, 2002, 28(1), 13–15 CrossRef.
- M. B. Dreifke, A. A. Jayasuriya and A. C. Jayasuriya, Mater. Sci. Eng., C, 2015, 48, 651–662 CrossRef CAS PubMed.
- A. Kasuya and Y. Tokura, J. Dermatol. Sci., 2014, 76, 169–172 CrossRef PubMed.
- H. F. Selig, D. B. Lumenta, M. Giretzlehner, M. G. Jeschke, D. Upton and L. P. Kamolz, Burns, 2012, 38, 960–966 CrossRef PubMed.
- L. I. F. Moura, A. M. A. Dias, E. Carvalho and H. C. de Sousa, Acta Biomater., 2013, 9, 7093–7114 CrossRef CAS PubMed.
- R. Xu, G. Luo, H. Xia, W. He, J. Zhao, B. Liu, J. Tan, J. Zhou, D. Liu, Y. Wang, Z. Yao, R. Zhan, S. Yang and J. Wu, Biomaterials, 2015, 40, 1–11 CrossRef CAS PubMed.
- Y. Wang, R. Xu, G. Luo, Q. Lei, Q. Shu, Z. Yao, H. Li, J. Zhou, J. Tan, S. Yang, R. Zhan, W. He and J. Wu, Acta Biomater., 2016, 30, 246–257 CrossRef CAS PubMed.
- J. L. Lowery, N. Datta and G. C. Rutledge, Biomaterials, 2010, 31, 491–504 CrossRef CAS PubMed.
- P. I. Morgado, P. F. Lisboa, M. P. Ribeiro, S. P. Miguel, P. C. Simões, I. J. Correia and A. Aguiar-Ricardo, J. Membr. Sci., 2014, 469, 262–271 CrossRef CAS.
- D. Liang, Z. Lu, H. Yang, J. Gao and R. Chen, ACS Appl. Mater. Interfaces, 2016, 8, 3958–3968 Search PubMed.
- Y. Wang, R. Xu, W. He, Z. Yao, H. Li, J. Zhou, J. Tan, S. Yang, R. Zhan, G. Luo and J. Wu, Tissue Eng., Part C, 2015, 21, 932–944 CrossRef CAS PubMed.
- P. I. Morgado, A. Aguiar-Ricardo and I. J. Correia, J. Membr. Sci., 2015, 490, 139–151 CrossRef CAS.
- B. S. Smith, S. Yoriya, T. Johnson and K. C. Popat, Acta Biomater., 2011, 7, 2686–2696 CrossRef CAS PubMed.
- Y. Dong, P. Li, C. Chen, Z. Wang, P. Ma and G. Chen, Biomaterials, 2010, 31, 8921–8930 CrossRef CAS PubMed.
- A. Hansson, N. Hashom, F. Falson, P. Rousselle, O. Jordan and G. Borchard, Carbohydr. Polym., 2012, 90, 1494–1500 CrossRef CAS PubMed.
- W. He, Z. Yao, Y. Huang, G. Luo and J. Wu, Burns & Trauma, 2014, 2, 196 Search PubMed.
- J. C. Jang, R. Choi, S. Han, S. Jeong and W. Kim, Ann. Plast. Surg., 2015, 74, 501–507 CrossRef CAS PubMed.
- Y. Wang, Z. Chen, G. Luo, W. He, K. Xu, R. Xu, Q. Lei, J. Tan, J. Wu and M. Xing, ACS Appl. Mater. Interfaces, 2016, 8, 7411–7421 CAS.
- J. Tan, X. Peng, G. Luo, B. Ma, C. Cao, W. He, S. Yuan, S. Li, J. A. Wilkins and J. Wu, PLoS One, 2010, 5, e9995 Search PubMed.
- Q. Zhang, H. Lu, N. Kawazoe and G. Chen, Acta Biomater., 2014, 10, 2005–2013 CrossRef CAS PubMed.
- J. L. Lowery, N. Datta and G. C. Rutledge, Biomaterials, 2010, 31, 491–504 CrossRef CAS PubMed.
- S. Schreml, R. Szeimies, L. Prantl, M. Landthaler and P. Babilas, J. Am. Acad. Dermatol., 2010, 63, 866–881 CrossRef PubMed.
- A. J. Singer and R. A. Clark, N. Engl. J. Med., 1999, 341, 738–746 CrossRef CAS PubMed.
- J. Scholz, G. Nocke, F. Hollstein and A. Weissbach, Surf. Coat. Technol., 2005, 192, 252–256 CrossRef CAS.
- T. Szabó, J. Mihály, I. Sajó, J. Telegdi and L. Nyikos, Prog. Org. Coat., 2014, 77, 1226–1232 CrossRef.
- F. Reyes-Ortega, A. Cifuentes, G. Rodríguez, M. R. Aguilar, Á. González-Gómez, R. Solis, N. García-Honduvilla, J. Buján, J. García-Sanmartin, A. Martínez and J. S. Román, Acta Biomater., 2015, 23, 103–115 CrossRef CAS PubMed.
- H. Y. Mi, X. Jing, M. R. Salick, T. M. Cordie and L. S. Turng, J. Mech. Behav. Biomed. Mater., 2016, 62, 417–427 CrossRef CAS PubMed.
- I. P. Kaur, S. K. Sandhu, P. K. Deol, G. Sharma, M. Yadav and M. Singh, Curr. Pharm. Des., 2015, 21, 1556–1574 CrossRef CAS PubMed.
- A. J. Reuvers, J. W. A. van den Berg and C. A. Smolders, J. Membr. Sci., 1987, 34, 45–65 CrossRef CAS.
- Y. D. Kim, J. Y. Kim, H. K. Lee and S. C. Kim, J. Appl. Polym. Sci., 1999, 73, 2377–2384 CrossRef CAS.
- D. M. Koenhen, M. H. V. Mulder and C. A. Smolders, J. Appl. Polym. Sci., 1977, 21, 199–215 CrossRef CAS.
- J. G. Wijmans, J. P. B. Baaij and C. A. Smolders, J. Membr. Sci., 1983, 14, 263–274 CrossRef CAS.
- L. Zeng, Y. Yao, D. A. Wang and X. Chen, Mater. Sci. Eng., C, 2014, 34, 168–175 CrossRef CAS PubMed.
- G. D. Winter, J. Wound Care, 1995, 4, 366–367 CAS.
- S. E. Mutsaers, J. E. Bishop, G. McGrouther and G. J. Laurent, Int. J. Biochem. Cell Biol., 1997, 29, 5–17 CrossRef CAS PubMed.
Footnote |
| † The two authors contribute equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |