Selective production of methanol by the electrochemical reduction of CO2 on boron-doped diamond electrodes in aqueous ammonia solution†
Prastika K. Jiwantia,
Keisuke Natsuia,
Kazuya Nakatab and
Yasuaki Einaga*ac
aDepartment of Chemistry, Keio University, 3-14-1 Hiyoshi, Yokohama 223-8522, Japan. E-mail: einaga@chem.keio.ac.jp
bPhotocatalysis International Research Center, Tokyo University of Science, 2641 Yamazaki, Noda, Chiba 278-8510, Japan
cACCEL, Japan Science and Technology Agency, 5-3 Yonbancho, Chiyoda 102-8666, Japan
First published on 18th October 2016
Abstract
The electrochemical reduction of CO2 was investigated in an aqueous ammonia solution using boron-doped diamond electrodes. Methanol was mainly produced by reduction at a potential of −1.3 V (vs. Ag/AgCl) with a faradaic efficiency as high as 24.3%. Also, even in an aqueous ammonium bicarbonate solution (pH 7.9) without CO2 bubbling, methanol was produced, while no methanol production was observed at higher (10.6) and lower pH (3.38). These observations suggest that the selectivity for methanol production in aqueous ammonia solutions is due to the electrochemical reduction of bicarbonate ions which are formed by the reaction between ammonia and CO2. Moreover, we present the important role of ammonia as an electrolyte for the selective production of methanol by electrochemical reduction of CO2.
Introduction
CO2 reduction is an important next-generation technology and has been widely studied by researchers, since it emphasizes the value of processes by producing chemicals or fuel. Although the high thermodynamic stability of CO2 makes its reduction difficult in general, efficient and suitable processes must be achieved. One useful strategy is to develop suitable catalysts, for example, the Ru(II)–Re(I) supramolecular photocatalyst,1 Re(CO)3(bpy)Cl complexes,2 and the Ag(II) porphyrin complex.3 On the other hand, several studies have also been reported on direct electrochemical reduction using metal electrodes,4 electrodes with some modifications,5 and carbon-based electrodes.6–9 However, a high overpotential is needed for direct electron transfer to the CO2 molecule (eqn (1)).
CO2 + e− → CO2˙−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E0 = −1.9 V (vs. NHE) E0 = −1.9 V (vs. NHE)
| (1) |
Thus, hydrogen evolution, which also occurs together with CO2 reduction, is a competing reaction, resulting in a high production of hydrogen gas.10,11
Conductive boron-doped diamond (BDD) is a material of great interest due to its superior electrochemical properties, such as wide potential window, low background current, chemical inertness, and mechanical durability, and it has been used for many electrochemical applications.12–14 The wide potential window and conductive behavior of BDD electrodes are suitable for supporting CO2 reduction in aqueous solutions and minimizing hydrogen evolution. In fact, we reported on the electrochemical reduction of CO2 using BDD electrodes in methanol, seawater, and NaCl solutions, producing formaldehyde and formic acid with high faradaic efficiency.15
On the other hand, carbon dioxide capture and storage (CCS) technology is one of the promising technologies for reducing carbon emissions. Amine solutions, such as monoethanolamine (MEA) and diethanolamine (DEA), are used for CCS technology, since they are chemically strong CO2 absorbers. In addition, ammonia (NH3) solution is also known as a strong absorber and has high loading capacity for CO2 scrubbing systems (3 times higher than MEA solutions).16–18
Along these lines, in this present work, the electrochemical reduction of CO2 on a BDD electrode was performed in weakly alkaline aqueous NH3 solution, resulting in the selective production of methanol.
Experimental
Chemicals
NH4HCO3 was purchased from Sigma Aldrich, and other reagents were purchased from Wako Pure Chemical Industries. All reagents were used without any further purification. Ultrapure water was obtained from a Symply-Lab water system (Direct-Q UV3, Millipore).Preparation of the BDD electrodes
BDD films were deposited onto Si (111) wafers using a microwave plasma-assisted chemical vapor deposition system (Model AX 5400, CORNES Technology Corp.). Details of the preparation are described elsewhere.14 A mixture of trimethoxyborane and acetone was used as the carbon and boron sources to achieve a B/C ratio of 1%.Electrochemical measurements
The electrochemical measurements were conducted in two-compartment cell separated by a Nafion membrane with 100 mL of solution in each cell (Fig. S1†). BDD, Pt mesh, and Ag/AgCl were used as a working electrode, a counter electrode, and a reference electrode, respectively. BDD with an area of 4.9 cm2 and the Ag/AgCl electrode were immersed on the catholyte side (1 M NH3), and the Pt mesh electrode was on the anolyte side (0.1 M NaCl). The BDD and Pt electrodes were pretreated by ultrasonication in ultrapure water. Ultrasonication in acetone and ultrapure water was also performed after each reduction process to clean the electrodes. Before reduction started, N2 gas was purged for 30 minutes to remove oxygen gas in the solution, followed by CO2 gas bubbling for 2 hours with a flow rate of 200 sccm. Cyclic voltammograms were taken before and after gas bubbling with a scan rate of 100 mV s−1. Electrochemical reduction of CO2 was performed by chronoamperometry for 2 hours at potentials ranging from −1.2 V to −1.5 V (vs. Ag/AgCl) at the room temperature and the atmospheric pressure. To study the mechanism, the reduction of an aqueous ammonium bicarbonate (NH4HCO3) solution was performed without CO2 bubbling. All electrochemical measurements were recorded using a potentiostat (Autolab PGSTAT204, Metrohm Autolab B.V.).Products analysis
The liquid products were analyzed using a gas chromatography-mass spectrometry (GCMS-QP2010 Ultra, Shimadzu Corp.) by the headspace method using ethanol as the internal standard. Meanwhile, the gas products were analyzed by gas chromatography with a flame ionization detector and a thermal conductivity detector (GC-2014, Shimadzu Corp.).Results and discussion
Fig. 1 shows cyclic voltammograms (CVs) on a BDD electrode in 1 M NH3 aqueous solution. After nitrogen gas purging, no reduction peak was observed for potentials ranging from 0 V to −1.8 V (vs. Ag/AgCl) (Fig. 1a). After CO2 gas was bubbled into the solution at a flow rate of 200 sccm for 2 hours, still no specific reduction peak was observed. However, a cathodic current was increased from around −1.0 V (vs. Ag/AgCl) (Fig. 1b).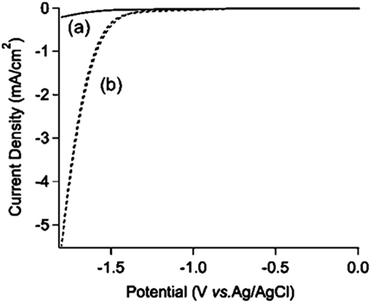 | ||
| Fig. 1 CVs on a BDD electrode in 1 M NH3 aqueous solution after nitrogen gas purging ((a) solid line) and after CO2 bubbling for 2 hours ((b) dashed line) with a scan rate of 100 mV s−1. | ||
After CO2 saturation (2 hours bubbling) in 1 M NH3 aqueous solution, the electrochemical reduction was performed for 2 hours at various potentials at room temperature and atmospheric pressure. The products after electrolysis at −1.3 V (vs. Ag/AgCl) were methanol (0.25 mg L−1, faradaic efficiency: 24.3%), carbon monoxide (0.002 mg L−1, faradaic efficiency: 0.05%), methane (0.0006 mg L−1, faradaic efficiency: 0.13%), and hydrogen (0.04 mg L−1, faradaic efficiency: 19.7%). Here, the dependence of the faradaic efficiency on the applied potential is summarized in Table 1. When the applied potential was −1.3 V (vs. Ag/AgCl), the efficiency of the methanol production was the highest. At the same time, the efficiencies for the production of CO and CH4 were quite low. Thus, methanol can be selectively produced by CO2 reduction on a BDD electrode in an aqueous NH3 solution. On the other hand, when the applied potential was more negative, the faradaic efficiency of the hydrogen evolution increased and that of the methanol production diminished.
| Potential (V vs. Ag/AgCl) | Faradaic efficiency (%) | |||
|---|---|---|---|---|
| CH3OH | CO | CH4 | H2 | |
| −1.2 | 2.61 | 0 | 0.24 | 0 |
| −1.3 | 24.3 | 0.05 | 0.13 | 19.7 |
| −1.4 | 15.1 | 0.12 | 0.03 | 25.8 |
| −1.5 | 2.02 | 0.20 | 0 | 57.2 |
The pH was monitored during CO2 bubbling in the 1 M NH3 aqueous solution. The initial pH was 11.7. During CO2 bubbling for 2 hours, the pH decreased to 7.7 (Fig. 2). In aqueous solutions, NH3 and CO2 react as follows (eqn (2)–(4)):16
NH3 + CO2 + H2O ⇄ NH4+ + HCO3− ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Keq.(293) = 1.02 × 103 Keq.(293) = 1.02 × 103
| (2) |
NH3 + HCO3− ⇄ NH2CO2− + H2O ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Keq.(293) = 3.61 Keq.(293) = 3.61
| (3) |
NH3 + HCO3− ⇄ CO32− + NH4+ ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Keq.(293) = 1.04 × 10−1 Keq.(293) = 1.04 × 10−1
| (4) |
 | ||
| Fig. 2 pH of the aqueous ammonia solution during CO2 gas bubbling. The measurement was conducted while stirring the solution at ∼25 °C. After 2 hours bubbling, pH decreased to 7.7. | ||
On the other hand, according to the distribution of carbonaceous species in the aqueous solution, the pH determines which species are dominant in the solution. It is known that dissolved CO2 (aq) is the dominant species at pH < 5, while at pH levels from 7.5 to 9, HCO3− (bicarbonate ion) is dominant, and CO32− (carbonate ion) is dominant above pH 12. The reaction is generally denoted as follows (eqn (5) and (6)):19
CO2 (aq) + H2O (l) ⇄ H+ (aq) + HCO3− (aq) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pK1 = 6.35 pK1 = 6.35
| (5) |
H+ (aq) + HCO3− (aq) ⇄ 2H+ (aq) + CO32− (aq) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pK2 = 10.33 pK2 = 10.33
| (6) |
Since the reaction of aqueous NH3 with CO2 rapidly produces bicarbonate (pH 7.6–8.0) after CO2 saturation, it is assumed that bicarbonate ions are reduced to methanol in our system.
In order to confirm that bicarbonate ions are reducible species, the electrochemical reduction of a 0.1 M NH4HCO3 aqueous solution (pH 7.9) was performed without CO2 bubbling. As the result, methanol was mainly produced in common with the reduction in an aqueous NH3 solution saturated with CO2 (Fig. 3).
In addition, we investigated into the influence of pH by adding HCl for low pH (<4) and NaOH for high pH (>10) to the NH4HCO3 solution. As the results, methanol was not detected in either condition at the same reduction potential of −1.3 V (vs. Ag/AgCl). These results suggest that bicarbonate ions are reducible species, not CO2 and CO32−. That is, we propose the mechanism of methanol production in our system as follows (eqn (7)):
| HCO3− + 5H2O + 6e− → CH3OH + 7OH− | (7) |
On the other hand, we considered the effect of NH3 on methanol production by the electrochemical reduction of bicarbonate ions. The electrochemical reduction was performed in 0.1 M KOH solution saturated with CO2 (pH 7.0) and in 0.1 M NaOH solution saturated with CO2 (pH 7.2) at a potential of −1.3 V (vs. Ag/AgCl) for 2 hours on a BDD electrode, resulting in no methanol production. It can be explained by the buffering effect of the solution. During the electrochemical reduction, the hydroxide ions (OH−) were produced by the reduction of bicarbonate ions and also by the hydrogen evolution. Therefore, the local pH will become slightly higher. However, in the aqueous NH3 solution, OH− reacts with ammonium ions to form ammonia and water by the reaction (eqn (8)):
| NH4+ + OH− ⇄ NH3 + H2O | (8) |
On the other hand, in the KOH and NaOH solution, the bicarbonate ions will react with OH− to form carbonate ions by the following reaction (eqn (9)):
| HCO3− + OH− ⇄ CO32− + H2O | (9) |
As mentioned previously, the reducible species are bicarbonate ions, and carbonate ions could not be reduced to methanol. Therefore, the presence of NH3 is important for methanol production. In addition, ammonia has the advantage of the higher loading capacity of CO2 than other aqueous solutions (Tables S1 and S2†). However, a clear and complete mechanism will need further study.
For comparison, the electrochemical reduction of CO2 on glassy carbon electrode was conducted at a potential of −1.3 V (vs. Ag/AgCl) for 2 hours. However, no methanol was detected, and most of the product was H2 reaching an amount of 0.26 mg L−1 (on the BDD electrode, it was 0.04 mg L−1). Therefore, methanol can be selectively produced by CO2 reduction on a BDD electrode, as long as the hydrogen evolution can be suppressed. This kind of continuous electrochemical reduction at quite high potential may cause surface corrosion of the glassy carbon electrode (emphasizing roughness, changing pores volume, etc.).19,20 On the other hand, BDD has high durability. In order to show evidence for the durability of BDD, the surface morphology of a BDD electrode was examined by SEM after using the BDD electrode for more than 30 hours in a reduction process. The SEM images revealed no difference as compared to the as-grown BDD (Fig. 4). This is consistent with the results in our previous report.15
Conclusions
We found that methanol can be selectively produced by the electrochemical reduction of CO2 in an aqueous ammonia solution on BDD electrodes at the potential of −1.3 V (vs. Ag/AgCl) with the faradaic efficiency as high as 24.3%. The methanol production is most likely due to the electrochemical reduction of bicarbonate ions which are formed by the reaction between ammonia and CO2. In addition, BDD electrodes show the high durability and the suppression of hydrogen evolution for the electrochemical reduction of CO2, compared with a glassy carbon electrode. This study shows the possibility for the practical applications on reducing industrial stored-CO2 (HCO3−), which usually use amine-based solutions for CO2 scrubbing systems.Acknowledgements
Prastika Krisma Jiwanti acknowledges the graduate scholarship from Indonesia Endowment Fund for Education (LPDP), Republic of Indonesia.Notes and references
- A. Nakada, K. Koike, K. Maeda and O. Ishitani, Green Chem., 2016, 18, 139 RSC
.
- J. M. Smieja and C. P. Kubiak, Inorg. Chem., 2010, 49, 9283 CrossRef CAS PubMed
.
- J. Y. Becker, B. Vainas, R. Eger and L. Kaufman, J. Chem. Soc., Chem. Commun., 1985, 1471 RSC
.
- S. Kaneco, K. Iiba, K. Ohta, T. Mizuno and A. Saji, Electrochim. Acta, 1998, 44, 573 CrossRef CAS
.
- M. Ma, K. Djanashvili and W. A. Smith, Phys. Chem. Chem. Phys., 2015, 17, 20861 RSC
.
- B. R. Eggins, E. M. Brown, E. A. McNeill and J. Grimshaw, Tetrahedron Lett., 1988, 29, 945 CrossRef CAS
.
- P. A. Christensen, A. Hamnett, A. V. G. Muir and N. A. Freeman, J. Electroanal. Chem. Interfacial Electrochem., 1990, 288, 197 CrossRef CAS
.
- K. Hara, A. Kudo and T. Sakata, J. Electroanal. Chem., 1997, 421, 1 CrossRef CAS
.
- N. Yang, S. R. Waldvogel and X. Jiang, ACS Appl. Mater. Interfaces, 2015 DOI:10.1021/acsami.5b09825
.
- T. Yamamoto, D. A. Tryk, K. Hashimoto, A. Fujishima and M. Okawa, J. Electrochem. Soc., 2000, 147, 3393 CrossRef CAS
.
- R. M. Hernández, J. Márquez, O. P. Márquez, M. Choy, C. Ovalles, J. J. Garcia and B. Scharifker, J. Electrochem. Soc., 1999, 146, 4131 CrossRef
.
- W. T. Wahyuni, T. A. Ivandini, P. K. Jiwanti, E. Saepudin, J. Gunlazuardi and Y. Einaga, Electrochemistry, 2015, 83, 357 CrossRef CAS
.
- Y. Einaga, J. Appl. Electrochem., 2010, 40, 1807 CrossRef CAS
.
- T. Yano, D. A. Tryk, K. Hashimoto and A. Fujishima, J. Electrochem. Soc., 1998, 145, 1870 CrossRef CAS
.
- K. Nakata, T. Ozaki, C. Terashima, A. Fujishima and Y. Einaga, Angew. Chem., Int. Ed., 2014, 53, 871 CrossRef CAS PubMed
.
- F. Mani, M. Peruzzini and P. Stoppioni, Green Chem., 2006, 8, 995 RSC
.
- S. Y. Park, K. B. Yi, C. H. Ko, J.-H. Park, J.-N. Kim and W. H. Hong, Energy Fuels, 2010, 24, 3704 CrossRef CAS
.
- H. Bai and A. C. Yeh, Ind. Eng. Chem. Res., 1997, 36, 2490 CrossRef CAS
.
- H. Zhong, K. Fujii, Y. Nakano and F. Jin, J. Phys. Chem. C, 2015, 119, 55 CAS
.
- G. M. Swain, J. Electrochem. Soc., 1994, 141, 3382 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: The apparatus for electrochemical reduction, pH measurements, CO2 concentration, and SEM images. See DOI: 10.1039/c6ra20466j |
| This journal is © The Royal Society of Chemistry 2016 |


