MgO-templated mesoporous carbons using a pitch-based thermosetting carbon precursor
Abstract
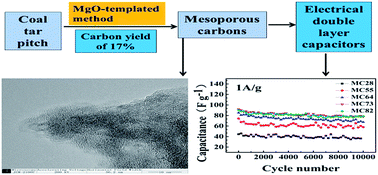
A pitch-based thermosetting amphiphilic carbonaceous material (CP-A5) was converted to mesoporous carbons (MCs) by an MgO-templated method. The yields of MCs were competitive, up to 17%. Temperature-programmed desorption (TPD), thermogravimetric analysis (TG), high-resolution transmission electron microscopy (HRTEM) and N2 adsorption–desorption were applied to characterize the precursors and MgO-templated MCs. Compared with some published template carbons from thermoplastic materials, less collapse was observed due to the thermosetting property of the carbon precursor. MC73 (MgO/CP-A5 at 7/3 mass ratio) had a surface area of 1991 m2 g−1 and interconnected micropores/mesopores, which endowed it with outstanding electrochemical performance in 1 M TEABF4/PC organic electrolyte, namely, a gravimetric specific capacitance of 90.8 F g−1 at 0.05 A g−1 and a capacitance retention of 91.3% after 10 000 cycles at 1 A g−1. MC73 is a promising electrode material candidate for energy storage by electrical double layer capacitors. Considering their competitive carbon yields as well as the cyclic ability of MgO, development of MCs from coal tar pitch by an MgO-templated method is feasible and promising, particularly in terms of the upgrade of heavy residues in coal-related industry.

 Please wait while we load your content...
Please wait while we load your content...