Effect of NOx on product yields and Arrhenius parameters of gas-phase oxidation of β-ocimene initiated by OH˙ radicals†
Elizabeth Gaona-Colmána,
María B. Blancoa,
Ian Barnesb and
Mariano A. Teruel*a
aInstituto de Investigaciones en Fisicoquímica de Córdoba (I. N. F. I. Q. C.), Facultad de Ciencias Químicas, Universidad Nacional de Córdoba. Ciudad Universitaria, 5000 Córdoba, Argentina. E-mail: mteruel@fcq.unc.edu.ar
bBergische Universität Wuppertal, Fakultät für Mathematik und Naturwissenschaften, Physikalische Chemie & Theoretische Chemie, Gauss Strasse 20, 42119 Wuppertal, Germany
First published on 19th September 2016
Abstract
Rate coefficients for the gas-phase reaction of OH˙ radicals with β-ocimene were measured using the relative rate method over the temperature range 288–311 K at 760 Torr total pressure of nitrogen. The experiments were performed in a large volume environmental chamber using long-path FTIR spectroscopy to monitor the reactants. A room temperature rate coefficient of k(β-ocimene+OH˙)= (2.36 ± 0.54) × 10−10 cm3 molecule−1 s−1 was obtained for the title reaction. The temperature dependent rate coefficients are best fit by the Arrhenius expression k= (4.02 ± 0.71) × 10−14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(2567 ± 211)/T. In addition, product studies have been performed at (298 ± 2) K and 760 of Torr of synthetic air in the absence and presence of NOx. The following molar products were determined: formaldehyde (16.5 ± 0.9)% and (24.3 ± 1.5)%, acetone (45.6 ± 2.1)% and (58.3 ± 3.4)%, methyl vinyl ketone (18.5 ± 0.8)% and <5% and glycolaldehyde (7.6 ± 0.6)% and <5% in the absence and presence of NOx, respectively. Acetic acid (<5%) was only found in the reaction performed in the absence of NOx. With NOx peroxy acetyl nitrate was formed with a yield <5%. Reaction mechanisms accounting for the formation of the products are proposed and atmospheric implications are discussed.
exp(2567 ± 211)/T. In addition, product studies have been performed at (298 ± 2) K and 760 of Torr of synthetic air in the absence and presence of NOx. The following molar products were determined: formaldehyde (16.5 ± 0.9)% and (24.3 ± 1.5)%, acetone (45.6 ± 2.1)% and (58.3 ± 3.4)%, methyl vinyl ketone (18.5 ± 0.8)% and <5% and glycolaldehyde (7.6 ± 0.6)% and <5% in the absence and presence of NOx, respectively. Acetic acid (<5%) was only found in the reaction performed in the absence of NOx. With NOx peroxy acetyl nitrate was formed with a yield <5%. Reaction mechanisms accounting for the formation of the products are proposed and atmospheric implications are discussed.
1. Introduction
According to emission inventories many organic compounds are emitted into the atmosphere from vegetation, whereby isoprene and monoterpenes are the main compounds.1 β-Ocimene ((E,Z)-3,7-dimethyl-1,3,6-octatriene) is an acyclic monoterpene and emissions have been detected from plants such as wheat,2 pine,3 grass land4 and tropical forests.5 In the troposphere, biogenic volatile organic compounds (BVOCs) may react with atmospheric oxidants such as OH˙ radicals, NO3 radicals and O3 or can be degraded by photolysis, processes that lead to the formation of a wide variety of product species and contribute to the formation of tropospheric ozone and particulate matter.6The gas-phase kinetics of the OH˙ radical-initiated degradation of β-ocimene has been studied previously by Atkinson et al.7 at 294 K and by Grimsrud et al.8 at 301 K, using in both cases the relative kinetic technique. In addition, this reaction has been studied as a function of temperature over the range 313–423 K by Kim et al.9 also using the relative kinetic technique.
Many product studies have been carried out under atmospheric conditions on the reactions of terpenes with the atmospheric oxidants (mainly with OH˙ radicals and O3 molecules), the results of which indicate the formation of polyfunctional product compounds.10–13 The products of the reaction of the OH˙ radical with β-ocimene have been studied by Reissell et al.11,13 in the presence of NO. The experiments were performed in a 7900 L Teflon chamber using multiple analysis techniques, i.e. gas chromatography (GC) with flame ionization detection (GC-FID), GC-mass spectrometry (GC-MS) and GC-Fourier transform infrared (GC-FTIR). They observed formation of acetone and 4-methylhexa-3,5-dienal with molar yields of 18–20% and <2%, respectively. The non-observation of the co-product of acetone (4-methyl-3,5-hexadienal), was attributed to the reaction of the intermediate 1,2-hydroxyalkoxy radicals formed by OH˙ radical addition at the –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)2 moiety in the compound with O2 or more likely their isomerization through five- or seven-membered transition states forming multifunctional products and some acetone. The alternative explanation was that the reaction of CH2
C(CH3)2 moiety in the compound with O2 or more likely their isomerization through five- or seven-membered transition states forming multifunctional products and some acetone. The alternative explanation was that the reaction of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(CH3)
CH(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2C(˙)HOH radicals with O2 does not result in the formation of 4-methyl-3,5-hexadienal. These authors have also performed products studies on the reaction of OH˙ radicals with myrcene, who is structurally similar to β-ocimene. They identified acetone and the expected co-product 4-vinyl-4-pentenal as products with higher molar yields of 36–45% and 19%, respectively.
CH2C(˙)HOH radicals with O2 does not result in the formation of 4-methyl-3,5-hexadienal. These authors have also performed products studies on the reaction of OH˙ radicals with myrcene, who is structurally similar to β-ocimene. They identified acetone and the expected co-product 4-vinyl-4-pentenal as products with higher molar yields of 36–45% and 19%, respectively.
In this work, rate coefficients for the reaction of the OH˙ radical with β-ocimene have been measured over the temperature range 288–311 K using the relative rate method. This investigation extends the previous kinetic temperature dependent study of Kim et al.9 to lower temperatures as in tropospheric environments since Kim et al.9 have reported rate coefficients as a function of temperature over the temperature range of 313–423 K.
In addition, in this study we present a product distribution of the gas-phase reaction of OH˙ radicals with β-ocimene in the presence and in the absence of NOx at 298 K and 760 Torr. To the best of our knowledge this is the first reported experimental product investigation of the OH-initiated oxidation for β-ocimene in the absence of NOx. In contrast to the previous product studies of this reaction our investigation has been able to identify products which eluded detection in the other studies. The measured product distribution is used to propose a more comprehensive atmospheric chemical degradation mechanism for the reaction than previously possible using the older product investigations.
2. Experimental section
The experiments were performed in a 1080 L chamber over the temperature range 288–311 K in 760 Torr of nitrogen for the kinetic experiments and synthetic air for product studies. The reactor consists of two cylindrical quartz glass vessels, each 3 m in length and 45 cm inner diameter, joined in the middle with both opened ends closed by aluminium flanges. The metal flanges contain ports for the introduction of bath gases and reactants into the reactor. Three fans with Teflon blades are mounted inside the chamber, which ensure the uniform mixing of the reactants. The reactor can be evacuated by a pumping system, consisting of a turbomolecular pump backed by a double stage rotary fore pump to 10−3 Torr. The reactor is equipped with 32 super actinic fluorescent lamps (Philips TL 05/40 W: 320 < λ < 480 nm, λmax = 360 nm) and 32 low-pressure mercury lamps (Philips TUV/40 W, λmax = 254 nm) which are spaced evenly around the reaction vessel. The lamps are wired in parallel and can be switched individually. The low-pressure mercury lamps were employed for the experiments.A white-type mirror system is mounted internally in the chamber and coupled to a FTIR spectrometer Thermo Nicolet Nexus. The spectrometer is equipped with a liquid nitrogen cooled mercury–cadmium–telluride (MCT) detector, which allows “in situ” monitoring of the reactants in the infrared range 4000–700 cm−1. The “white” mirror system in the reactor was operated with the total optical absorption path length set to 484.7 m. Infrared spectra were recorded with a spectral resolution of 1 cm−1. Typically, 60 interferograms for OH˙ reaction were co-added per spectrum and 15 such spectra were recorded per experiment. The chamber is described in greater detail in Barnes et al.14,15 The reactor is equipped with a system for temperature regulation over the range 284–313 K with a precision of ±1 K.
Rate coefficients for the reaction of OH˙ radicals with β-ocimene were determined by comparing their rate of decay with that of the corresponding decay of reference compounds:
| OH˙ + β-ocimene → products, kocimene | (1) |
| OH˙ + reference → products, kreference | (2) |
Provided that the reference compound and the reactant are lost only by reactions (1) and (2), then it can be shown that:
 | (I) |
Hydroxyl radicals were generated from the 254 nm photolysis of hydrogen peroxide (H2O2). The relative rate technique relies on the assumption that the β-ocimene and reference compound are removed solely by reaction with the OH˙ radicals. To verify this assumption, the concentrations of β-ocimene and the reference compounds were monitored, in the absence of H2O2, in the dark and also with the photolysis lamps switched on for time periods equivalent to those used for the experiments. No significant changes of the β-ocimene and reference compound concentrations were observed. 2,3-Dimethyl-2-butene with a rate coefficient of kOH = (1.09 ± 0.04) × 10−10 cm3 molecule−1 s−1 (ref. 16) was used as a reference compound for the determination of the rate coefficient of OH˙ with β-ocimene at 298 K. Isobutene was employed as reference compound for the temperature dependent studies using the Arrhenius expression k = 0.947 × 10−11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(504/T−1) cm3 molecule−1 s−1 to calculate the rate coefficients for the appropriate temperature.6,17
exp(504/T−1) cm3 molecule−1 s−1 to calculate the rate coefficients for the appropriate temperature.6,17
To identify and to quantify the products formed in the reaction of OH˙ radical with β-ocimene, mixtures of H2O2/β-ocimene/air (with and without NO) were irradiated for periods of 16–20 min during the course of which infrared spectra were recorded with the FTIR spectrometer. Typically 60 interferograms were co-added per spectrum over a period of approximately 1 min and 20 such spectra were collected.
Reactants and products were quantified by comparison with calibrated reference spectra contained in the IR spectral databases of the laboratory in Wuppertal. The reactants were monitored at the following infrared absorption frequencies (in cm−1): β-ocimene 2700–3150; 2,3-dimethyl-2-butene 2800–3050; isobutene 890. The products observed were monitored at the following infrared spectra (in cm−1): acetone 1217; methyl vinyl ketone 952; glycolaldehyde 1764; formaldehyde 2766; acetic acid 1184 and peroxy acetyl nitrate 1163.
The initial concentrations of the organic compounds (1 ppmV = 2.46 × 1013 molecule cm−3 at 298 K) were: 2.7–3 ppmV for β-ocimene; 2.3–2,6 ppmV for 2,3-dimethyl-2-butene; 1.9–2 ppmV for isobutene; ∼7 ppmV for H2O2 and 3 ppmV for NO. The chemicals used in the experiments had the following purities as given by the manufacturer and were used as supplied: nitrogen (Air Liquide, 99.999%), nitric oxide (Messer Griesheim, 99.5%), hydrogen peroxide (Peroxid-Chemie GmbH, 85%), β-ocimene mixture of cis/trans isomers (Aldrich, ≥90%), 2,3-dimethyl-2-butene (Aldrich, 98%) and isobutene (Messer Griesheim, 99%).
3. Results and discussion
3.1. Room temperature rate coefficient
Fig. 1 shows the kinetic data, plotted according to eqn (I), obtained from experiments on the reaction of the OH˙ radical with β-ocimene at 298 K measured relative to the reference compounds 2,3-dimethyl-2-butene and isobutene. Each plot represents three experiments for each reference compound and as can be seen linear relationships were obtained for both reference compounds. Listed in Table 1 are the rate coefficient ratios kocimene/kreference obtained from the kinetic plots and the corresponding rate coefficients for the reaction of OH˙ with β-ocimene put on an absolute basis using the rate coefficient values of the reference compound given in the Experimental section. Since all the rate coefficient values obtained for the reaction of OH˙ with β-ocimene from the individual experiments using two reference compounds are in excellent agreement we prefer to quote a final value for the rate coefficient which is the average of all the determined values. This results in the following final averaged value for the reaction at 298 K:| k(β-ocimene+OH˙)= (2.36 ± 0.54) × 10−10 cm3 molecule−1 s−1 |
 | ||
| Fig. 1 Plot of the kinetic data for the reaction of the OH˙ radical with β-ocimene measured relative to (O) 2,3-dimethyl-2-butene and (□) isobutene at 298 ± 2 K and atmospheric pressure of N2. | ||
| Reaction | Reference | kocimene/kreference | kocimene × 1010 (cm3 molecule−1 s−1) |
|---|---|---|---|
| β-Ocimene + OH˙ | 2,3-Dimethyl-2-butene | 2.038 ± 0.039 | 2.22 ± 0.13 |
| 2,3-Dimethyl-2-butene | 2.333 ± 0.058 | 2.54 ± 0.16 | |
| 2,3-Dimethyl-2-butene | 2.335 ± 0.054 | 2.55 ± 0.16 | |
| Isobutene | 4.753 ± 0.105 | 2.44 ± 0.54 | |
| Isobutene | 4.253 ± 0.084 | 2.19 ± 0.48 | |
| Isobutene | 4.316 ± 0.066 | 2.22 ± 0.48 | |
| Average: | 2.36 ± 0.54 |
The above value for the reaction of OH˙ with β-ocimene measured in this work at 298 K can be compared with other reported literature values. Kim et al.9 have reported a value of (3.03+0.44−0.39) × 10−10 cm3 molecule−1 s−1 for the reaction at 298 K measured relative to OH˙ with isobutene. They performed the investigations in a 160 cm3 quartz reaction chamber using GC-MS for the analysis. The value of the rate coefficient at 298 K quoted by the authors is derived from their Arrhenius expression. Atkinson et al.7 report a value of (2.50 ± 0.19) × 10−10 cm3 molecule−1 s−1 for the reaction at 294 K measured relative to 2,3-dimethyl-2-butene. Their study was carried out in 6400 L all-Teflon chamber using CG/FID for the analysis. Finally, Grimsrud et al.8 report a value of 3.2 × 10−10 cm3 molecule−1 s−1 for the reaction at 301 K measured relative to isobutene. The value of the rate coefficient obtained in this work for the reaction at 298 K of (2.36 ± 0.54) × 10−10 cm3 molecule−1 s−1 is in excellent agreement with the value reported by Atkinson et al.7 and agrees within the reported error limits with that reported by Kim et al.9 Our value is 36% lower than the value of 3.2 × 10−10 cm3 molecule−1 s−1 reported by Grimsrud et al.8
Linear free-energy relationships are often used to estimate rate coefficients for the reactions of compounds with atmospheric oxidants which have not yet been the subject of experimental determination. These correlations are based on the assumption that the electrophilic addition of oxidants such as OH˙ radicals, O(3P) atoms, NO3 radicals, O3 molecules and Cl atoms to unsaturated compounds proceed via similar reaction mechanisms and, therefore, the rate coefficients for the reactions should be well correlated with one another.18–21 In this work, we present the correlation between kOH and kO3 for a series of acyclic alkenes and terpenes containing a conjugated double bound moiety. Table 2 lists the recommended room temperature rate coefficients for the reactions of different alkenes and terpenes with OH˙ radicals and O3 molecules. Fig. 2 shows a plot of the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kO3 values versus the corresponding log
kO3 values versus the corresponding log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kOH˙ values. As seen in Fig. 2 a reasonable correlation is obtained. A least-squares treatment of the data (r2 = 0.89) yields the following expression (rate coefficients in units of cm3 molecule−1 s−1):
kOH˙ values. As seen in Fig. 2 a reasonable correlation is obtained. A least-squares treatment of the data (r2 = 0.89) yields the following expression (rate coefficients in units of cm3 molecule−1 s−1):
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kO3 = (3.15 ± 0.38) log kO3 = (3.15 ± 0.38) log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kOH˙ + (14.75 ± 3.75) kOH˙ + (14.75 ± 3.75) |
| Row | Compounds | kOH˙ × 1010 (cm−3 molecule−1 s−1) | kO3 × 1017 (cm−3 molecule−1 s−1) |
|---|---|---|---|
| 1 | CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 1,3-butadiene CH2 1,3-butadiene |
0.666 (ref. 36) | 0.624 (ref. 36) |
| 2 | CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)C(CH3) C(CH3)C(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 2,3-dimethyl-1,3-butadiene CH2 2,3-dimethyl-1,3-butadiene |
1.25 (ref. 36) | 2.62 (ref. 36) |
| 3 | CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH3 1,3-pentadiene CHCH3 1,3-pentadiene |
1.03 (ref. 36) | 2.78 (ref. 36) |
| 4 | CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 1,3,5-hexatriene CH2 1,3,5-hexatriene |
1.04 (ref. 36) | 2.62 (ref. 36) |
| 5 | CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)CH C(CH3)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 isoprene CH2 isoprene |
1.11 (ref. 36) | 1.30 (ref. 36) |
| 6 | CH3C(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2CH CHCH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)CH C(CH3)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 β-ocimene CH2 β-ocimene |
2.36 (this work) | 38.5 (ref. 9) |
| 7 | CH2C(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2CH2C( CHCH2CH2C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)CH CH2)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 β-myrcene CH2 β-myrcene |
3.35 (ref. 9) | 38.5 (ref. 9) |
| 8 | CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC( CHC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)CH2CH2CH CH2)CH2CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)CH2CH2CH C(CH3)CH2CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)CH3 β-farnesene C(CH3)CH3 β-farnesene |
2.88 (ref. 9) | 68.6 (ref. 9) |
| 9 | CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(CH3) CHC(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2CH CHCH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)CH2CH2CH C(CH3)CH2CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)CH3 α-farnesene C(CH3)CH3 α-farnesene |
2.19 (ref. 9) | 59.4 (ref. 9) |
 | ||
Fig. 2 Linear free energy plot of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kO3 against log kO3 against log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kOH˙ at room temperature for a series of acyclic alkenes and terpenes with a conjugated double bound system. See Table 2 for the number interpretations. kOH˙ at room temperature for a series of acyclic alkenes and terpenes with a conjugated double bound system. See Table 2 for the number interpretations. | ||
The good correlation supports that the mechanism of the reactions of the OH˙ radical with the selected acyclic poly-alkenes and terpenes is similar to that for the reactions of the compounds with O3 molecules, i.e. the addition of OH˙ or O3 to the double bonds of the unsaturated compounds in a reversible step forming an adduct, which can react in subsequent fast reactions forming products.7,17 Using the correlation obtained in this work, reasonably precise estimations can be made of rate coefficients for reactions of OH˙ or O3 with acyclic poly-unsaturated compounds which have not yet been determined experimentally.
3.2. Dependence of the rate coefficient on temperature
The temperature dependence of the reaction of OH˙ radical with β-ocimene was studied over the temperature range 288–311 K using isobutene as the reference compound. Fig. 3 shows the kinetic data obtained from the experiments performed at 288, 293, 298, 303 and 311 K plotted according to eqn (I). Good linear correlations were obtained at all temperatures. The rate coefficients for the reaction of OH˙ with β-ocimene, derived from linear least-squares analysis of each experimental data set at the individual temperatures investigated, are listed in Table 3. The errors quoted for the rate coefficients are twice the standard deviation arising from the straight line least-squares fits of the data, plus an additional 20% to take into account uncertainties in the rate coefficient for isobutene. An Arrhenius plot of the kinetic data is shown in Fig. 4. The following Arrhenius expression was has been derived from a linear least-squares treatment of the plot:k (OH˙ + β-ocimene) = (4.02 ± 0.71) × 10−14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp((2567 ± 211)/T) exp((2567 ± 211)/T) |
| Reaction | Temperature | kocimene × 1010 (cm3 molecule−1 s−1) | −Ea/R (K) | A × 1014 (cm3 molecule−1 s−1) |
|---|---|---|---|---|
| β-Ocimene + OH˙ | 288 K | 2.95 ± 0.68 | 2567 ± 211 | 4.02 ± 0.71 |
| 293 K | 2.53 ± 0.56 | |||
| 298 K | 2.36 ± 0.54 | |||
| 303 K | 1.87 ± 0.44 | |||
| 311 K | 1.54 ± 0.36 |
The rate coefficients for the reaction of OH˙ with β-ocimene were found to decrease with increasing temperature over the temperature range 288–311 K. Kim et al.9 also observe negative temperature dependence for the reaction and this is plotted in Fig. 4 together with the other two room temperature values of the rate coefficient obtained by Grimsrud et al.8 and Atkinson et al.7 The negative temperature of the reaction observed in this work is much stronger than that observed by Kim et al.9 our value of Ea/R is a factor of 4 higher than their value for the reaction.
The negative temperature dependence of the reaction of OH˙ with β-ocimene can be justified on a qualitative basis assuming that the lifetime of the excited bimolecular complex, formed between the OH˙ radical and the terpene, with respect to decomposition back to the reactants decreases as the temperature increases. The probability of the excited adduct being stabilized by collision with a third body decreases with increasing temperature. Alternately, one can treat a termolecular reaction as the sum of individual bimolecular reactions, which result in an effective activation energy for the global reaction, i.e. the overall activation energy is the sum of the energies for the individual steps. The effective activation energy for the reaction is negative and the rate coefficient decreases as the temperature increases when the activation energy for the energized complex back to reactants is greater than the sum of those for its formation and reactions to form products.22
3.3. Product studies
Formation of acetone, methyl vinyl ketone, formaldehyde, glycolaldehyde and acetic acid was observed in the experiments performed in the absence of NO and acetone, methyl vinyl ketone, formaldehyde, glycolaldehyde and peroxy acetyl nitrate was observed in the experiments performed in the presence of NOx. Concentration–time profiles of β-ocimene and the identified products in the absence and presence of NO are presented in Fig. S3 and S4,† respectively. The concentration–time profiles support that acetone, methyl vinyl ketone, glycolaldehyde, formaldehyde and acetic acid are to a large extend primary products in the absence of NO and acetone, methyl vinyl ketone, glycolaldehyde, formaldehyde and peroxy acetyl nitrate are to a large extend primary products in presence of NO. This linearity does not, however, exclude contributions to some of the products from secondary reactions.
Plots of the concentrations of acetone, methyl vinyl ketone, formaldehyde, glycolaldehyde and acetic acid, as a function of consumed β-ocimene all show reasonable linearity. Such plots for the products formed in the OH˙ + β-ocimene reaction in the absence of NO are shown in Fig. S5† and the corresponding plots for the products formed in the presence of NO in Fig. S6.† The reactions of OH˙ with formaldehyde, methyl vinyl ketone and glycolaldehyde have IUPAC23 recommended 298 K rate coefficients values (in cm3 molecule−1 s−1) of 8.5 × 10−12, 2.0 × 10−11 and 8.0 × 10−12, respectively. Formaldehyde, methyl vinyl ketone and glycolaldehyde will be subject to secondary consumption by OH˙ in the reaction system. To estimate the extent of secondary consumption by OH˙ for these compounds the formation yield correction method outlined in Tuazón et al.24 was used. The calculated secondary consumption in the reaction system for these carbonyl compounds was less than 1% in all cases and has, therefore, not been taken into consideration in the reported formation yields. The formation yields of the oxidation products identified in the OH-radical initiated oxidation of β-ocimene in the presence and absence of NO are listed in Table 4.
| Reaction | Product | Yield (%) (3 ppmV NOx) | Yield (%) (NOx-free) |
|---|---|---|---|
| β-Ocimene + OH˙ | HC(OH) formaldehyde | 24.3 ± 1.5 | 16.5 ± 0.9 |
| CH3C(O)CH3 acetone | 58.3 ± 3.4 | 45.6 ± 2.1 | |
CH3C(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 methyl vinyl ketone CH2 methyl vinyl ketone |
<5% | 18.5 ± 0.8 | |
| HOCH2C(O)H glycolaldehyde | <5% | 7.6 ± 0.6 | |
| CH3C(O)OONO2 peroxy acetyl nitrate (PAN) | <5% | — | |
| CH3C(O)OH acetic acid | — | <5% |
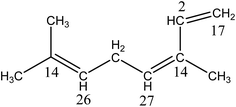
The sample of β-ocimene investigated was a racemic mixture of the cis and trans isomers the chemical structures of which are shown below:
The molecular structure of β-ocimene contains isobutene ((CH3)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–) and isoprene (–CH
C–) and isoprene (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)–CH
C(CH3)–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) type moieties. The reaction of OH˙ radicals with β-ocimene proceeds overwhelmingly by addition of OH˙ to the three double bonds in the molecule to form hydroxyalkyl radicals, which react rapidly under the experimental conditions with oxygen to form hydroxy peroxy radicals. The structure–reactivity relationship of Kwok and Atkinson25 predicts that ∼62% of the OH˙ addition will occur at the –CH
CH2) type moieties. The reaction of OH˙ radicals with β-ocimene proceeds overwhelmingly by addition of OH˙ to the three double bonds in the molecule to form hydroxyalkyl radicals, which react rapidly under the experimental conditions with oxygen to form hydroxy peroxy radicals. The structure–reactivity relationship of Kwok and Atkinson25 predicts that ∼62% of the OH˙ addition will occur at the –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)–CH
C(CH3)–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 moiety and ∼38% at the (CH3)2C
CH2 moiety and ∼38% at the (CH3)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– moiety with an negligible contribution from H-atom abstraction pathways. The SAR of Peeters et al.26 for (poly)alkenes not only predicts a similar OH˙ addition percentage for the two double bond moieties but also gives estimations of the site-specific contributions of OH˙ addition to the double bonds. The OH˙-radical site specific addition estimates, as percentages of the overall rate coefficient, are shown below:
CH– moiety with an negligible contribution from H-atom abstraction pathways. The SAR of Peeters et al.26 for (poly)alkenes not only predicts a similar OH˙ addition percentage for the two double bond moieties but also gives estimations of the site-specific contributions of OH˙ addition to the double bonds. The OH˙-radical site specific addition estimates, as percentages of the overall rate coefficient, are shown below:
Addition of OH˙ at the isobutene moiety will lead to formation of two 1,2-hydroxy peroxy radicals. Due to resonance between the two double bond units in the isoprene moiety in β-ocimene formation of six different hydroxyperoxy radicals is possible, i.e. four 1,2-hydroxyperoxy and two 1,4-hydroxyperoxy radicals which can have E and Z configurations.
In the presence of NO, at the levels used in the experiments, the hydroxyperoxy radicals will be mainly converted to hydroxyalkoxy radicals, however, formation of hydroxynitrates is also possible. In absence of NO the hydroxyperoxy radicals will undergo self-reactions or reactions with other peroxy radicals (HO2/RO2) to form to a large extend hydroxyalkoxy radicals, although molecular channels are also possible.27,28 Reactions of the hydroxyperoxy radicals with HO2 radicals could potentially lead to the formation of hydroxy-hydroperoxides. At the radical levels present in the experiments it is very unlikely that isomerization of the hydroxyl peroxy radicals, as reported for the reaction of OH˙ with isoprene,29 will occur. The hydroxyalkoxy radicals can react further with molecular oxygen or decompose by C–C scissions. In the case of β-ocimene, isomerization can also occur through five or six membered transition states, leading to the formation of multifunctional products that will still contain >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C< moieties.13
C< moieties.13
In this study, the yields of acetone obtained in the absence and presence of NOx are (45.6 ± 2.1)% and (58.3 ± 3.4)%, respectively. The main route to the formation of acetone is attributed to decomposition of the CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(CH3)
CHC(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2CH(OH)C(O˙)(CH3)CH3 and CH2
CHCH2CH(OH)C(O˙)(CH3)CH3 and CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(CH3)
CHC(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2CH(O˙)C(OH)(CH3)CH3 radicals. These radicals are generated by addition of the OH radical to the double bond between C6 and C7 (see channel A1 and channel A2 in Fig. S7†). The yields of acetone are ∼8% and ∼20% higher, for the absence and presence of NOx, respectively, than the ∼38% estimated fraction of the overall OH˙ radical reaction performing by initial OH˙-radical addition to the –CH
CHCH2CH(O˙)C(OH)(CH3)CH3 radicals. These radicals are generated by addition of the OH radical to the double bond between C6 and C7 (see channel A1 and channel A2 in Fig. S7†). The yields of acetone are ∼8% and ∼20% higher, for the absence and presence of NOx, respectively, than the ∼38% estimated fraction of the overall OH˙ radical reaction performing by initial OH˙-radical addition to the –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)2 moiety. Tuazón et al.30 have observed high yields of acetone in their study on the reaction of OH radicals with 2-methylpropene, 2-methyl-2-butene and 2,3-dimethyl-2-butene in the presence of NOx. Each of these alkene contains an >C
C(CH3)2 moiety. Tuazón et al.30 have observed high yields of acetone in their study on the reaction of OH radicals with 2-methylpropene, 2-methyl-2-butene and 2,3-dimethyl-2-butene in the presence of NOx. Each of these alkene contains an >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C entity and Tuazón et al.30 found that for each alkene bond cleavage of the intermediate hydroxyalkoxy radicals to form acetone in yields of ∼90% dominated over reaction of the radicals with O2. The high yields of acetone observed in this study for the reaction OH˙ with β-ocimene support that the main fate of the hydroxyalkoxy radicals formed by addition of the OH˙ radical to the double bond between C6 and C7 will also be bond cleavage to form acetone and that the alternative reaction pathways of the radicals with O2 and possibly also isomerization are insignificant.
C entity and Tuazón et al.30 found that for each alkene bond cleavage of the intermediate hydroxyalkoxy radicals to form acetone in yields of ∼90% dominated over reaction of the radicals with O2. The high yields of acetone observed in this study for the reaction OH˙ with β-ocimene support that the main fate of the hydroxyalkoxy radicals formed by addition of the OH˙ radical to the double bond between C6 and C7 will also be bond cleavage to form acetone and that the alternative reaction pathways of the radicals with O2 and possibly also isomerization are insignificant.
It is quite probable that there is some secondary formation of acetone in the reaction systems through reactions of OH˙ with reaction products that contain the >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C entity. In Fig. S6,† although a straight line has been drawn through the measurements points to obtain the yield, closer inspection of the last 4 points in the plot suggest that some upward curvature is occurring, which would be indicative of secondary acetone formation. Secondary formation of acetone could explain the much higher yield of acetone observed in the presence of NOx compared to that in its absence where the yield is more in accord with the calculated fraction of the reaction proceeding via OH˙ radical addition to the >C
C entity. In Fig. S6,† although a straight line has been drawn through the measurements points to obtain the yield, closer inspection of the last 4 points in the plot suggest that some upward curvature is occurring, which would be indicative of secondary acetone formation. Secondary formation of acetone could explain the much higher yield of acetone observed in the presence of NOx compared to that in its absence where the yield is more in accord with the calculated fraction of the reaction proceeding via OH˙ radical addition to the >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C entity. A comparison of the ocimene decay in Fig. S3 and S4† shows that the decay of ocimene is much faster in the presence of NOx due to the higher levels of OH˙ which are generated by the NOx chemistry in the system thus causing a faster ocimene decay and consequently more secondary formation of acetone.
C entity. A comparison of the ocimene decay in Fig. S3 and S4† shows that the decay of ocimene is much faster in the presence of NOx due to the higher levels of OH˙ which are generated by the NOx chemistry in the system thus causing a faster ocimene decay and consequently more secondary formation of acetone.
Reissell et al.11,13 have determined the yield of acetone in the OH˙-radical initiated oxidation of ocimene in the presence of NO at room temperature and 740 Torr total pressure of air in large Teflon chambers using GG/FID and CG/MS to detect acetone. In these studies they report acetone molar formation yields of only 18% and 20%. The yield of acetone in the presence of NOx obtained in this work is a factor of ∼3 higher than the yields reported by Reissell et al.11,13 Curiously, Reissell et al.13 report a yield of 45% for acetone formation in the OH˙-radical initiated oxidation of the structurally similar myrcene. Reissell et al.13 offer various explanations for the much lower acetone yield they observe in the reaction of OH˙ with ocimene compared to myrcene, however, we have performed extensive compound calibration and spectra subtraction checks to confirm our yields and suspect that the low acetone yields obtained by Reissell et al.11,13 has other, as yet unknown, reasons. Reissell et al.11 report that ocimene reacts in the dark with NO2, however, inspection of the dark period in our product study on the reaction of OH˙ with ocimene in the presence of NOx (see Fig. S4†) shows that under our conditions reaction of ocimene with NO2 was negligible. Based on the results of Reissell et al.11 any significant interference by reaction of NO2 with ocimene in our experimental system would have caused a decrease in the acetone yield over the NOx-free case rather than the significant enhancement which we observe.
Formaldehyde has been identified and quantified with a yield of (24.3 ± 1.5)% in the presence of NOx and (16.5 ± 0.9)% in absence of NOx. According to the mechanisms presented in Fig. S7–S9,† formaldehyde could be formed by decomposition of the radicals CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(CH3)
CHC(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2CH(OH)C(O˙)(CH3)CH3, ˙OCH2CH(OH)C(CH3)
CHCH2CH(OH)C(O˙)(CH3)CH3, ˙OCH2CH(OH)C(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2CH
CHCH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)CH3 and CH2
C(CH3)CH3 and CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O˙) (CH3)CH(OH)CH2CH
CHC(O˙) (CH3)CH(OH)CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)CH3 generated by addition of the OH radical at C6, C1 and C4, respectively (see channel A2 in Fig. S7,† channel A1 in S8 and channel A2 in S9). No other yield for the formation of formaldehyde from the reaction of β-ocimene + OH˙ has been reported in the literature. However, a yield of (30 ± 6)% has been reported for formaldehyde formation in the reaction of OH˙ with myrcene by Orlando et al.,12 which it is structurally similar to β-ocimene, they performed their study in the presence of NOx in a stainless steel environmental chamber using long-path FTIR for the analysis. Taking into account the structural similarity between of β-ocimene and myrcene and consequently, the similarity in the degradation pathways with OH˙ radicals, the yield of formaldehyde obtained in this work in the presence of NOx is in relatively good agreement with the value obtained by Orlando et al.12
C(CH3)CH3 generated by addition of the OH radical at C6, C1 and C4, respectively (see channel A2 in Fig. S7,† channel A1 in S8 and channel A2 in S9). No other yield for the formation of formaldehyde from the reaction of β-ocimene + OH˙ has been reported in the literature. However, a yield of (30 ± 6)% has been reported for formaldehyde formation in the reaction of OH˙ with myrcene by Orlando et al.,12 which it is structurally similar to β-ocimene, they performed their study in the presence of NOx in a stainless steel environmental chamber using long-path FTIR for the analysis. Taking into account the structural similarity between of β-ocimene and myrcene and consequently, the similarity in the degradation pathways with OH˙ radicals, the yield of formaldehyde obtained in this work in the presence of NOx is in relatively good agreement with the value obtained by Orlando et al.12
Glycolaldehyde was formed with a yield of (7.6 ± 0.6)% in the absence of NOx and <5% in the presence of NOx. We suggest that this product is formed by decomposition of the HOCH2CH(O˙)C(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2CH
CHCH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)CH3 radical which results when the OH˙ radical adds to the C1 position (see channel B1 in Fig. S8†). Formation of methyl vinyl ketone has been observed with yields of (18.5 ± 0.8)% in the absence of NOx and <5% in the presence of NOx. This product is probably formed according to the mechanism shown in Fig. S9,†i.e. through decomposition of the CH2
C(CH3)CH3 radical which results when the OH˙ radical adds to the C1 position (see channel B1 in Fig. S8†). Formation of methyl vinyl ketone has been observed with yields of (18.5 ± 0.8)% in the absence of NOx and <5% in the presence of NOx. This product is probably formed according to the mechanism shown in Fig. S9,†i.e. through decomposition of the CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O˙)(CH3)CH(OH)CH2CH
CHC(O˙)(CH3)CH(OH)CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)CH3 radical formed by the addition of the OH˙ radical to the C4 position (see channel A1 Fig. S9†). In addition, formation of acetic acid was observed in the absence of NOx with a yield of <5%, and it is suggested that it is formed by decomposition of the CH3CH(O˙)(OH)CH
C(CH3)CH3 radical formed by the addition of the OH˙ radical to the C4 position (see channel A1 Fig. S9†). In addition, formation of acetic acid was observed in the absence of NOx with a yield of <5%, and it is suggested that it is formed by decomposition of the CH3CH(O˙)(OH)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 radical. This radical is generated by the decomposition of the hydroxylalkoxy radical formed by channel B1 shown in Fig. S9.† Formation of acetic acid was not observed in experiments carried out in the presence of NOx.
CH2 radical. This radical is generated by the decomposition of the hydroxylalkoxy radical formed by channel B1 shown in Fig. S9.† Formation of acetic acid was not observed in experiments carried out in the presence of NOx.
The results obtained in this study indicate that acetone is a major product of the OH˙-radical initiated degradation of β-ocimene both in the absence and presence of NOx. Therefore, the addition of the OH˙ radical to the double bond between C6 and C7 and the following decomposition of the intermediate hydroxyalkoxy radicals (see channel A1 and B1 in Fig. S7†) are the main degradation pathways for the studied reaction both in the presence and the absence of NOx. The formation of methyl vinyl ketone is more favored in the absence of NOx. This is contrary to expectations since one would expect that the formation of the hydroxyalkoxy radical, which leads to formation of the ketone (channel A in Fig. S9†), would be more efficiently generated in the presence of NO. The hydroxyalkoxy radical generated in the presence of NO will be chemically activated and it would appear that this may cause the radical to react by other pathways such as decomposition or isomerization leading to the formation of an oxygenated compound other than methyl vinyl ketone. Also the formation of glycolaldehyde is slightly favored in the absence of NOx over the presence of NOx, the difference, however, is not very significant.
The formation of 4-methyl-3,5-hexadienal, methyl-vinyl-ketone and acetone have been observed as major oxidation products during sunlight irradiation of ocimene/NOx mixtures31,32 which will involve both OH˙- and O3-mediated oxidation of ocimene. Unfortunately we have no information on product yields from the studies. Besides formaldehyde, methylglyoxal and malonaldehyde three higher aldehydes were also tentatively identified in small amounts.
Acknowledgements
We gratefully acknowledge the financial support for this research provided by the EU project EUROCHAMP2, CONICET (Argentina), SECyT (Argentina) and FONCyT (Argentina).References
- J. Kesselmeier and M. Staudt, J. Atmos. Chem., 1999, 33, 23–88 CrossRef CAS.
- R. G. Buttery, C. Xu and C. L. Ling, J. Agric. Food Chem., 1985, 33(1), 115–117 CrossRef.
- F. Loreto, P. Nasceti, A. Graverini and M. Mannozzi, Funct. Ecol., 2000, 14, 589–595 CrossRef.
- G. König, M. Brunda, H. Puxbaum, C. N. Hewitt, C. Duckham and J. Rudolph, Atmos. Environ., 1995, 29, 861–874 CrossRef.
- J. Kesselmeier, P. Ciccioli, U. Kuhn, P. Stefani, T. Biesenthal, S. Rottenberger, A. Wolf, M. Vitullo, R. Valentini, A. Nobre, P. Kabat and M. O. Andreae, Global Biogeochem. Cycles, 2002, 16(4), 1126 CrossRef.
- R. Atkinson and J. Arey, Atmos. Environ., 2003, 37(2), S197–S219 CrossRef CAS.
- R. Atkinson, S. M. Aschmann and J. N. Pitts Jr, Int. J. Chem. Kinet., 1986, 18, 287–299 CrossRef CAS.
- E. P. Grimsrud, H. H. Westberg and R. A. Rasmussen, Int. J. Chem. Kinet., 1975, 183–195 Search PubMed.
- D. Kim, P. S. Stevens and A. Hites, J. Phys. Chem. A, 2011, 115, 500–506 CrossRef PubMed.
- H. Hakola, J. Arey, S. M. Aschmann and R. Atkinson, J. Atmos. Chem., 1994, 18, 75–102 CrossRef CAS.
- A. Reissell, C. Harry, S. M. Aschmann, R. Atkinson and J. Arey, J. Geophys. Res., 1999, 104(D11), 13869–13879 CrossRef CAS.
- J. J. Orlando, B. Nozière, G. Tyndall, G. E. Orzechowska, S. E. Paulson and Y. Rudich, J. Geophys. Res., 2000, 105(D9), 11561–11572 CrossRef CAS.
- A. Reissell, S. M. Aschmann, R. Atkinson and J. Arey, J. Geophys. Res., 2002, 107(D12), 4138 CrossRef.
- I. Barnes, K. H. Becker and T. Zhou, J. Atmos. Chem., 1993, 17, 353–373 CrossRef CAS.
- I. Barnes, K. H. Becker and N. Mihalopoulos, J. Atmos. Chem., 1994, 18, 267–289 CrossRef CAS.
- R. Atkinson, S. M. Aschmann and W. P. Carter, Int. J. Chem. Kinet., 1983, 15, 1161–1177 CrossRef CAS.
- R. Atkinson, J. Phys. Chem. Ref. Data, 1997, 26, 215–290 CrossRef CAS.
- J. S. Gaffney and S. Z. Levine, Int. J. Chem. Kinet., 1979, 11, 1197–1209 CrossRef CAS.
- R. Atkinson, Chem. Rev., 1985, 85, 69–201 Search PubMed.
- D. Grosjean and E. L. Williams II, Atmos. Environ., 1992, 26, 1395–1405 CrossRef.
- M. B. Blanco, I. Bejan, I. Barnes, P. Wiesen and M. A. Teruel, Atmos. Environ., 2009, 43, 5996–6002 CrossRef CAS.
- B. J. Finlayson-Pitts and J. N. Pitts Jr, Chemistry of the Upper and Lower Atmosphere, Academic Press, New York, 2000 Search PubMed.
- IUPAC Task Group on Atmospheric Chemical Kinetic Data Evaluation, http://iupac.pole-ether.fr, 2007 Search PubMed.
- E. C. Tuazón, H. Mac Leod, R. Atkinson and W. P. L. Carter, Environ. Sci. Technol., 1986, 20, 383–387 CrossRef PubMed.
- E. S. C. Kwok and R. Atkinson, Atmos. Environ., 1995, 29, 1685–1695 CrossRef CAS.
- J. Peeters, W. Boullart, V. Pultau, S. Vandenberk and L. Vereecken, J. Phys. Chem. A, 2007, 111, 1618–1631 CrossRef CAS PubMed.
- P. D. Lightfoot, R. A. Cox, J. N. Crowley, M. Destriau, G. D. Hayman, M. E. Jenkin, G. K. Moortgat and F. Zabel, Atmos. Environ., 1992, 26, 1805–1964 CrossRef.
- J. J. Orlando and G. S. Tyndall, Chem. Soc. Rev., 2012, 41, 6294–6317 RSC.
- J. D. Crounse, F. Paulot, H. G. Kjaergaard and P. O. Wennberg, Phys. Chem. Chem. Phys., 2011, 13, 13607–13613 RSC.
- E. C. Tuazón, S. M. Aschmann, J. Arey and R. Atkinson, Environ. Sci. Technol., 1998, 32, 2106–2112 CrossRef.
- A. Calogirou, Ph.D. Thesis, The Technical University of Munich, Germany, 1997.
- A. Calogirou, B. R. Larsen and D. Kotzias, Atmos. Environ., 1999, 33, 1423–1439 CrossRef CAS.
- R. Hein, P. J. Crutzen and M. Heimann, Global Biogeochem. Cycles, 1997, 11, 43–76 CrossRef CAS.
- J. A. Logan, J. Geophys. Res., 1985, 90(D6), 10463–10482 CrossRef.
- Y. Shu and R. Atkinson, J. Geophys. Res., 1995, 100(D4), 7275–7281 CrossRef CAS.
- NIST Chemical Kinetics Database, Standard Reference Database 17, Version 7.0, Web Version, http://kinetics.nist.gov/kinetics/index.jsp Search PubMed.
- R. Atkinson, D. Hasegawa and S. M. Aschmann, Int. J. Chem. Kinet., 1990, 22, 871–887 CrossRef CAS.
- R. Atkinson, S. M. Aschmann, A. Winer and J. N. Pitts Jr, Environ. Sci. Technol., 1985, 19, 159–163 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra20321c |
| This journal is © The Royal Society of Chemistry 2016 |