In situ polymerization of a novel surfactant on a graphene surface for the stable dispersion of graphene in water†
Abstract
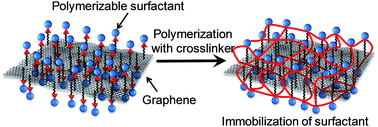
A pristine graphene surface is functionalized with a novel polymerizable surfactant by in situ polymerization with a crosslinker. The in situ polymerization immobilizes the surfactant molecules on the graphene surfaces, which allows the stable dispersion and redispersion of graphene in water. The resultant polymerized-surfactant/graphene is then shown to be useful as a conductive graphene ink.

 Please wait while we load your content...
Please wait while we load your content...