Monohydride signature as a key predictor of successful Si(110) surface functionalization
Abstract
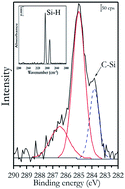
Methyl-terminated (110)-oriented silicon surfaces have been prepared from monohydride-terminated, H–Si(110) surfaces using a chlorination/alkylation procedure. Transmission infrared spectroscopy of the H–Si(110) surfaces showed absorption features indicating monohydride structures along the [−110] direction. X-ray photoelectron spectroscopy was used to characterize the methyl-terminated, CH3–Si(110), surfaces. Surface coverage calculations revealed 0.83 of an equivalent monolayer coverage for methyl-terminated Si(110) surfaces. No oxidation of silicon was observed in the high-resolution Si 2p spectra and the samples were stable against oxidation over time, with only 0.2 of a monolayer of surface oxide observed a month after the sample preparation. Thus, the chlorination/alkylation procedure can be used for the functionalization of monohydride Si(110) surfaces with improved long term stability.


 Please wait while we load your content...
Please wait while we load your content...