Hydralazine inhibits amyloid beta (Aβ) aggregation and glycation and ameliorates Aβ1–42 induced neurotoxicity
Abstract
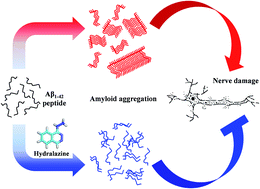
Alzheimer's disease (AD) is a neurodegenerative disorder affecting millions of people worldwide, characterized by senile plaques formed due to deposition of insoluble aggregates of amyloid beta (Aβ) peptide in neuronal synapses of the brain. The synthesis of the Aβ peptide and its aggregation is central to AD related pathogenesis. Post-translational modifications such as glycation, is known to exacerbate Aβ aggregation and neurodegeneration. Thus inhibitors of glycation can potentially inhibit Aβ aggregation and attenuate the progression of neurodegeneration. In the present study, we have evaluated the effect of hydralazine on Aβ aggregation and fibril formation by employing thioflavin-T (ThT), 1-anilino-8-naphthalene sulfonic acid (ANS) fluorescence assays, atomic force microscopy and static light scattering assay. From the results of these experiments, it is evident that hydralazine inhibits Aβ aggregation and fibril formation. Circular dichroism (CD) analysis revealed that hydralazine prevents β-sheet formation of Aβ peptide thereby inhibiting amyloid aggregation. Furthermore, molecular dynamics (MD) simulations studies also revealed that hydralazine binds to Aβ monomers as well as protofibrils and potentially destabilizes Aβ monomer–monomer interaction and protofibrils, thereby possibly alleviating Aβ neurotoxicity. This was evident by 3[4,5-dimethylthiazol-2-yl]2,5-diphenyl-tetrazolium bromide (MTT) assay, which confirmed that hydralazine ameliorates Aβ induced neuronal toxicity. This study suggests that hydralazine is a potential candidate for drug repositioning for the management of AD. However, it has to be re-engineered to reduce vasoactive effects and improve blood brain barrier permeability for future use in AD therapeutics.

 Please wait while we load your content...
Please wait while we load your content...