Catalytic performance of a series of guanidinium-based ionic liquids in the coupling reaction of carbon dioxide with epoxides†
Ping Li,
Ya Li,
Ci Chen,
Li Wang* and
Jinglai Zhang*
Institute of Environmental and Analytical Sciences, College of Chemistry and Chemical Engineering, Henan University, Kaifeng, Henan 475004, P. R. China. E-mail: chemwangl@henu.edu.cn; zhangjinglai@henu.edu.cn
First published on 6th September 2016
Abstract
In order to gain more insight into the high catalytic activity of N′′-(2-aminoethyl)-N,N,N′,N′-tetramethylguanidine bromide ([TMGC2H4NH2]Br) and to select a more suitable catalyst for the synthesis of propylene carbonate (PC), the cycloaddition reaction of carbon dioxide (CO2) into the epoxide (EO), catalyzed by a series of functional guanidinium-based ionic liquids (FGBILs), is schematically studied by the Density Functional Theory (DFT). The calculated results indicate that the formation of carbamic acid is a common pathway with a lower barrier height when the NH2-functionalized IL encounters CO2. What is more, the formation of carbamic acid is helpful in decreasing the barrier height of the ring-opening step. Two ILs are involved in the process of forming carbamic acid. Sparked by this, the mechanism catalyzed by two ILs is explored for comparison with the model catalyzed by one IL. In addition, the catalytic activities of other functionalized guanidinium-based ionic liquids are investigated, the results indicate that the task-specified ILs have better catalytic activity than those without functional groups because of the increased acidity. Besides the cation, the influence of different anions and substrates is also investigated.
1. Introduction
The utilization of CO2 has attracted continuous attention owing to the serious increase of global warming together with the decreasing availability of fossil fuels. Due to its high abundance, low cost, non-toxicity, and non-flammable nature, CO2 is very attractive as the C1 source for valuable chemicals.1–4 One promising conversion of CO2 into useful chemicals is its insertion into epoxides (EO) to form cyclic carbonates (PC).4–7 PC has wide applications as polar aprotic solvents, electrolytic elements of lithium secondary batteries, intermediates for organic and polymeric synthesis, and ingredients for pharmaceutical/fine chemicals in biomedical applications.6 Unfortunately, the activation of CO2 is difficult due to its kinetic inertness. Therefore, developing suitable and high-efficiency catalysts is necessary to surmount the obstacle for the fixation of CO2. Many catalytic systems have been developed for the cycloaddition of CO2. Representative catalysts include metal oxides,8,9 alkali metal salts,10,11 quaternary onium salts,12,13 ionic liquids (ILs),14–17 transition metal complexes,18–20 and functional organic compounds and polymers.21,22 Although the advances are significant, some of the catalysts still suffer from the disadvantages including the low catalytic reactivity/stability, harsh reaction condition, being water/air-sensitive, requirement of co-catalyst, toxicity issues, and combination of these features. Hence, the design of efficient, stable, and single component catalysts that facilitate the production of PC under benign condition is still desirable. So far, IL has attracted great interest owing to its good solvating ability, negligible vapor pressure, variable polarity, and other outstanding advantages.Since 1990s, various ILs, such as, quaternary ammonium,12,23,24 phosphonium,12,25,26 and imidazolium salts27–29 with excellent reactivity have been gradually developed. Except for the experimental effort, lots of theoretical investigations have been performed for the mechanism catalyzed by ionic liquids, such as, alkylmethylimidazolium chlorine ionic liquids ([Cnmim]Cl, n = 2, 4, and 6),30 1,3-dimethylimidazolium dibromobis(dimethylphosphato)zinc (DmimBr[Me2PO4] + ZnBr2),29 tetrabutylammonium bromide (TBABr),31,32 tetraethylammonium bromide (TEABr),32 tetraethylammonium chloride (TEACl),32 LiBr,33 1,5,7-triaza-bicyclo[4.4.0]dec-5-enium bromide (TBDHBr),34 KI/glycerol,35 tetrabutylammonium iodide/pyrogallol (TBAI/pyrogallol),36 Zn(salphen)/NBu4X (salphen = N,N′-bis(salicylidene)-1,2-phenylenediamine, X = Br, I),37 azaphosphatranes,38 perfluoro-tert-butanol/tetrabutylammonium bromide (PFB/TBABr),39 and hexaalkylguanidinium salt/zinc bromide.40 Later, the functional groups are introduced into the imidazolium-based ILs resulting in the higher activity, which have been studied in our previous works.23,41 Except for the hydrogen bonding or electrostatic interaction reported by others,30,32 possessing the lively proton that can easily transfer is one of the most important factors to decrease the barrier height.
Inspired by the previous work, Dai et al. firstly synthesized a series of functionalized guanidinium-based ILs (FGBILs) and employed them to produce the cyclic carbonate,42 including [TMGC2H4NH2]Br, N′′-(2-carboxylethyl)-N,N,N′,N′-tetramethylguanidine bromide ([TMGC2H4COOH]Br), N′′-(2-hydroxylethyl)-N,N,N′,N′-tetramethylguanidine bromide ([TMGC2H4OH]Br), N′′-propyl-N,N,N′,N′-tetramethylguanidine bromide ([TMGC2H4CH3]Br), and N′′-ethyl-N,N,N′,N′-tetramethylguanidine bromide ([TMGC2H5]Br). Moreover, [TMGC2H4NH2]Br, [TMGC2H4COOH]Br, and [TMGC2H4OH]Br present better catalytic activity than [TMGC2H4CH3]Br and [TMGC2H5]Br. What is the reason for the higher catalytic activity of a series of FGBILs? What is the mechanism for the coupling reaction of CO2 and EO catalyzed by NH2-functionalized guanidinium-based IL? Dai et al. proposed that the primary amine (R–NH2) first reacts with CO2 to form carbamic acid (R–NHCOOH). In the next step, the carbamic acid would react with EO. The existence of carbamic acid has been confirmed through the Fourier transform infrared (FT-IR) spectrum. What is the mechanism to form the carbamic acid? After formation of carbamic acid, what is the mechanism of the following steps? Is it the same or totally different as compared to hydroxyl and carboxyl-functionalized ILs? Is the formation of the carbamic acid rate-limiting step?
To answer the questions raised above and gain insight into the mechanism of the whole catalytic cycle, the detailed mechanisms of cycloaddition of CO2 catalyzed by a series of FGBILs are studied by means of density functional theory (DFT). Our general goals are to elucidate the mechanism in detail, to explore the role of different cations and/or anions, and to deeply understand the different/similar points between amino-guanidinium based ILs and hydroxyl- and carboxyl-guanidinium based ILs. To our best knowledge, rare studies have concerned about the guanidinium-based IL. We expect that the present studies will be helpful to not only understand the intrinsic properties of the cycloaddition reaction but also provide constructive clues for the design of new catalysts with better performance.
2. Computational details
All the DFT calculations are performed by the Gaussian 09 program.43 The combination of Becke's three-parameter hybrid exchange functional combined with the Lee–Yang–Parr correlation (B3LYP) with the 6-31G(d,p) basis set is employed to perform the geometric optimization, which is a popular and reliable method to study the mechanism.44–47 At the same level, the vibrational frequencies are calculated to confirm that the optimized structures are minima without imaginary frequency (NImag = 0) and transition states with only one imaginary frequency (NImag = 1). In addition, the zero-point energy (ZPE) correction is also derived. Starting from the transition states, the minimum-energy path (MEP) is constructed by the intrinsic reaction coordinate (IRC) theory to confirm that the transition state is connected with two desired minima.48 To refine the energy, the single-point energy corrections were performed by the B3LYP/6-311+G(2d,2p) level on the basis of the optimized geometries at the B3LYP/6-31G(d,p) level.44,49 Finally, the atomic charge distributions are calculated by natural bond orbital (NBO) analysis to better understand how catalysts control the coupling reaction.50,513. Results and discussion
3.1 Without catalyst
The reaction mechanism of EO with CO2 in the absence of catalyst has been studied in our previous work with the same functional and basis set.41 It is a concerted mechanism to complete the ring-opening and CO2 insertion in one step. The barrier height is as high as 59.79 kcal mol−1, which is difficult to overcome in benign condition. So the catalyst is necessary for incorporation of CO2 into EO. Ionic liquid, especially for the single-component task-specified ionic liquid, as one of the most efficient catalysts, has been widely applied in the synthesis of PC.3.2 Comparing the catalytic effect of [TMGC2H4COOH]Br (1b), [TMGC2H4OH]Br (1c), [TMGC2H4CH3]Br (1d), and [TMGC2H5]Br (1e)
According to the literatures and our previous work,30,41 there are two great modifications in the presence of the catalyst. One is that the barrier height is greatly decreased by 20–30 kcal mol−1. The other is that the mechanism is changed from the concerted mechanism in the absence of catalyst to the stepwise mechanism in the presence of catalyst. There are three pathways for the stepwise mechanism as presented in Fig. 1. The first pathway is the least favorable because of the higher energy required to activate the CO2.30,33,52 For other two pathways, the three-step mechanism is more favorable than two-step mechanism, which is attributed to the synergistic effects of the acid–base active sites. In pathway iii, the oxygen atom of the EO is activated by the acid from the functional group (–OH, –COOH, etc.) or from the guanidine group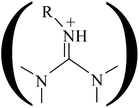 ; simultaneously the carbon atom of EO is motivated by the nucleophilic attack of Br− anion to form a ring-opening complex; subsequently, the CO2 reacts with the activated ring-opening substrate; finally, the cyclic carbonate is formed by intramolecular cyclization and the catalyst is regenerated.30,34,41,53 To compare the catalytic effect of ILs with different cations, only the most favorable reaction route, i.e., three-step mechanism, is calculated for every catalyst. The schematic energetic profiles and key structures are presented in Fig. 2. The structures of transition states and intermediates are plotted in Fig. S1 of ESI.† Cartesian coordinates of optimized reactants, intermediates, transition states, and product are listed in Table S1.† Seen from Fig. 2, three common points are found for routes 1–4. First, the ring-opening step is the rate-determining step, so activation of EO leading to a lower-energy intermediate is the essential factor in a whole catalytic cycle. Note that the energy of reactants is set to be zero for reference to obtain the relative energy, i.e., the values listed in Fig. 2. The rate-determining step is judged on the basis of the barrier height that is the difference between the energy of transition state and that of corresponding intermediate. The barrier heights of ring-opening step and ring-closure step are listed in Table 1. Second, the process of proton-transfer is involved in all of four catalysts. Third, the hydrogen bond is helpful not only to stabilize the substrate but also to activate the EO. Thus, the ring-opening is the cooperative result of hydrogen-bond and electrostatic interaction. The barrier height of rate-determining step increases in the following order, 1 < 2 <3 < 4, that is, the catalysts with functional group (1b and 1c) have better catalytic performance, which is attributed to the stronger acidity of task-specified ILs. In addition, the charges of the transferred hydrogen atoms in functional groups (0.505e in –COOH group for 1b and 0.488e in –OH group for 1c) are higher than ones in guanidine group (0.409e for 1d and 0.409e for 1e). The significant charge difference between the transferred hydrogen atom and the O atom of EO will induce the stronger columbic interaction resulting in the lower barrier heights and corresponding higher yields. Furthermore, the hydrogen bond is also an important factor to stabilize the transition state and decrease the barrier height. Owing to the stronger conjugation of 1b, the hydrogen bonding between carboxyl functional group and EO is stronger than that between hydroxyl group and EO. Hence, the catalytic activity of 1b is slightly higher than that of 1c, although they have almost the same acidity. It is also verified again that the ring-opening step is promoted by both the hydrogen bonding and the electrostatic interaction.
; simultaneously the carbon atom of EO is motivated by the nucleophilic attack of Br− anion to form a ring-opening complex; subsequently, the CO2 reacts with the activated ring-opening substrate; finally, the cyclic carbonate is formed by intramolecular cyclization and the catalyst is regenerated.30,34,41,53 To compare the catalytic effect of ILs with different cations, only the most favorable reaction route, i.e., three-step mechanism, is calculated for every catalyst. The schematic energetic profiles and key structures are presented in Fig. 2. The structures of transition states and intermediates are plotted in Fig. S1 of ESI.† Cartesian coordinates of optimized reactants, intermediates, transition states, and product are listed in Table S1.† Seen from Fig. 2, three common points are found for routes 1–4. First, the ring-opening step is the rate-determining step, so activation of EO leading to a lower-energy intermediate is the essential factor in a whole catalytic cycle. Note that the energy of reactants is set to be zero for reference to obtain the relative energy, i.e., the values listed in Fig. 2. The rate-determining step is judged on the basis of the barrier height that is the difference between the energy of transition state and that of corresponding intermediate. The barrier heights of ring-opening step and ring-closure step are listed in Table 1. Second, the process of proton-transfer is involved in all of four catalysts. Third, the hydrogen bond is helpful not only to stabilize the substrate but also to activate the EO. Thus, the ring-opening is the cooperative result of hydrogen-bond and electrostatic interaction. The barrier height of rate-determining step increases in the following order, 1 < 2 <3 < 4, that is, the catalysts with functional group (1b and 1c) have better catalytic performance, which is attributed to the stronger acidity of task-specified ILs. In addition, the charges of the transferred hydrogen atoms in functional groups (0.505e in –COOH group for 1b and 0.488e in –OH group for 1c) are higher than ones in guanidine group (0.409e for 1d and 0.409e for 1e). The significant charge difference between the transferred hydrogen atom and the O atom of EO will induce the stronger columbic interaction resulting in the lower barrier heights and corresponding higher yields. Furthermore, the hydrogen bond is also an important factor to stabilize the transition state and decrease the barrier height. Owing to the stronger conjugation of 1b, the hydrogen bonding between carboxyl functional group and EO is stronger than that between hydroxyl group and EO. Hence, the catalytic activity of 1b is slightly higher than that of 1c, although they have almost the same acidity. It is also verified again that the ring-opening step is promoted by both the hydrogen bonding and the electrostatic interaction.
 | ||
| Fig. 1 Possible pathways for the cycloaddition between epoxide and carbon dioxide catalyzed by FGBILs. | ||
 | ||
| Fig. 2 Potential energy profiles for the cycloaddition reaction along routes 1–4 calculated at the B3LYP/6-311+G(2d,2p)//B3LYP/6-31G(d,p) level. | ||
| Catalyst | Route | Ring-opening (kcal mol−1) | Ring-closure (kcal mol−1) |
|---|---|---|---|
| [TMGC2H4COOH]Br (1b) | Route 1 | 19.14 | 16.03 |
| [TMGC2H4OH]Br (1c) | Route 2 | 20.16 | 15.51 |
| [TMGC2H4CH3]Br (1d) | Route 3 | 22.77 | 15.99 |
| [TMGC2H5]Br (1e) | Route 4 | 23.02 | 15.90 |
| [TMGC2H4NH2]Br (1a) | Route 7 | 18.24 | 15.83 |
| [TMGC2H4NH2]BF4 (2a) | Route 8 | 28.40 | 6.41 |
| [TMGC2H4NH2]PF6 (3a) | Route 9 | 31.73 | 3.74 |
3.3 Mechanism in the presence of [TMGC2H4NH2]Br (1a)
According to the experimental result, the catalyst [TMGC2H4NH2]Br (1a) has the best catalytic performance among catalysts 1a–1e. To elucidate the reason for its significant catalytic effect, the essential thing is to explore the mechanism. It is no doubt that we should focus on the three-step mechanism and the ring-opening step.On the basis of previous experience, route 5 (see Fig. 3) is firstly established with the same mechanism as routes 1–4. The rupture of C–O bond in EO activated by both the hydrogen atom from guanidine group and the Br− anion is the rate-determining step. The following two steps, i.e., CO2 insertion and ring-closure to form PC, are not discussed anymore because of their minor importance and great similarity with other catalysts. The first barrier height of route 5 (22.40 kcal mol−1) is much higher than those of routes 1 and 2. Optimized structures of the intermediates and transition states are shown in Fig. S2.† It is contradictory with the higher catalytic activity of 1a reported in experiment. Since the O atom of EO can be activated by the hydrogen atom of catalyst, the hydrogen atom with more positive charge will be helpful to promote the ring opening. Will the hydrogen atom in –NH2 group play the better effect? It is unfortunate that this idea is failure to be realized. The NBO charges of two hydrogen atoms in –NH2 group are 0.388e and 0.380e, which are even lower than that of hydrogen atom in guanidine group. So the hydrogen atoms in –NH2 group have little possibility to activate the O atom of EO. It is also consistent with the experimental observation that there is no significant interaction between the amino group and EO.42
 | ||
| Fig. 3 Potential energy profiles for the cycloaddition reaction along routes 5–7 calculated at the B3LYP/6-311+G(2d,2p)//B3LYP/6-31G(d,p) level. | ||
To obtain the higher yield, other novel pathways are located to obtain the energy-rich substrate. One of the alternative pathways is that the primary amine will react directly with CO2 to form carbamic acid.42 Then, the carbamic acid will react with EO as a carbamic acid-functionalized IL. When the CO2 gets close to the N atom in –NH2 group of [TMGC2H4NH2]Br, a prereaction complex is formed barrierlessly with the energy of −0.85 kcal mol−1 (see route 6 in Fig. 3). Subsequently, the C atom of CO2 will attack the N atom of –NH2 group associated with the H1 transferring from N2 to O1 atom leading to the formation of carbamic acid (see the structures in Fig. 4). The barrier height of first step, i.e., forming carbamic acid, is as high as 43.03 kcal mol−1, which is impossible to complete. Moreover, it is even more difficult than route 5 in which no carbamic acid is formed in the catalytic cycle. However, the formation of carbamic acid has been identified by experimental measurement, which testifies to have another reaction route. In addition, it has been confirmed that forming the carbamic acid is a common process in the CO2 absorption.54–56 Sparked by the previous literatures, one more ionic liquid is considered in the process of forming carbamic acid. Two [TMGC2H4NH2]Br (1a) and one CO2 firstly form a tri-molecular complex via hydrogen bonds. Next, the addition of C1 to N2 atom is accompanied with the H1 shift from N2 to N3 atom. At the same time, the H2 migrates from N3 to O1 atom to form carbamic acid. The barrier height of ts7-1 is 11.03 kcal mol−1, which is easy to be completed at room temperature. The following steps are overlapped with those of route 6. The ring-opening step is still the rate-determining step with the barrier height of 18.24 kcal mol−1. The corresponding barrier heights of ring-opening step are listed in Table 1. It is obvious that the catalytic activity decreases in the order of 1a > 1b > 1c > 1d > 1e, which is consistent with the sequence of experimental measurement.
 | ||
| Fig. 4 Optimized geometries for the intermediates and transition states for the formation of TMGC2H4NHCOOH involved in routes 6–7. Distances are in angstroms. | ||
Based on the calculated results, there are three distinguishable features for all five catalysts 1a–1e. (1) The ring-opening process is the rate-determining step. So activating EO is the key step to improve the catalytic performance. (2) The ring-opening of EO is activated by the cooperative effect from both nucleophilic attack of anion and the acid motivation of cation. As a result, the catalyst with higher acidity presents better catalytic performance. (3) The task-specified IL has better catalytic activity because of including the hydrogen atom with more positive charge. We hope that these findings are helpful to understand the intrinsic properties of the reaction sequence and provide useful clues for the development of more powerful catalyst systems.
3.4 Mechanism in the presence of two [TMGC2H4NH2]Br (1a) ion pairs
In previous works the mechanism studies are all constructed by one catalyst molecule and one substrate. Is it good enough to describe the mechanism? Since two catalyst molecules are necessary to decrease the barrier height in the process of carbamic acid formation, are they also vital for activation of the ring-opening of EO? Except for formation of the carbamic acid, the ring-opening step of EO is also explored in the presence of two catalytic ion pairs. The key structures and schematic energy profile are presented in Fig. 5. The barrier height of the ring-opening step in route a is 16.36 kcal mol−1 that is lower than that of route 7 (18.24 kcal mol−1). However, the difference between two models is not fatal. In the view of structure, the EO is only activated by one carbamic acid, while the other carbamic acid is to stabilize the substrate. As a result, the model with one catalyst is suitable to qualitatively describe the mechanism. | ||
| Fig. 5 Potential energy profiles for the ring-opening step of routes a and 7 calculated at the B3LYP/6-311+G(2d,2p)//B3LYP/6-31G(d,p) level. | ||
3.5 Comparing the catalytic effect of [TMGC2H4NH2]Br (1a), [TMGC2H4NH2]BF4 (2a), and [TMGC2H4NH2]PF6 (3a)
Considering that the nucleophilic attack is the other essential factor to promote the ring-opening of EO, the catalytic activity of the catalysts with different anions is explored. Only the ring-opening step is studied and the corresponding results are plotted in Table 1. The barrier heights of ts8-2 and ts9-2 related with catalysts 2a and 3a, respectively, are much higher than that of ts7-2 related with catalyst 1a. It is consistent with the experimental measurement that the yields catalyzed by 2a and 3a are much lower than that catalyzed by 1a. Both the bulky structure and low electronegativity of the anion for 2a and 3a will decrease their nucleophilic ability.3.6 Catalytic activity toward different EOs
To refine the reaction condition, the catalytic activity of different reactants, 2-methyl-oxirane (Ra) and oxabicyclo[4.1.0]heptane (Rg) (route 10 shown in Fig. S3†), catalyzed by 1a is investigated. The mechanisms of different substrate with the same catalyst are the same. Moreover, the ring-opening step is still the rate-determining step. However, the barrier height of ts10-2 (28.51 kcal mol−1) is much higher than that of ts7-2 (18.24 kcal mol−1), which is consistent with the experimental result. The structure of two rings hinders the nucleophilic attack of Br− anion leading to the low yield of Rg. Selecting the suitable substrate is also one of the most key factors to refine the reaction.4. Conclusion
In this work, the mechanism of coupling reaction of CO2 is studied in the presence of a series of guanidinium-based ILs. The mechanism catalyzed by [TMGC2H4NH2]Br is elucidated. On the basis of above studies, we get the following conclusions:(1) Two amino-functionalized ILs will react with CO2 to form the carbamic acid firstly. The following steps are the same with other hydroxyl- or carboxyl-functionalized ILs.
(2) The ring-opening of the EO is activated by the cooperative effect of both the acid and the nucleophilic attack. To design a new catalyst with the higher activity, the following factors should be considered: one is to introduce more acidic functional group; the other is to choose anion with stronger ability of nucleophilic attack.
(3) The suitable substrate that is easy to be nucleophilic attacked is also an important factor to achieve the higher yields.
Acknowledgements
We thank the National Supercomputing Center in Shenzhen (Shenzhen Cloud Computing Center) for providing computational resources and software. This work was supported by the National Natural Science Foundation of China (21376063, 21476061) and Program for He'nan Innovative Research Team in University (15IRTSTHN005).References
- H. Arakawa, M. Aresta, J. N. Armour, M. A. Barteau, E. J. Beckman, A. T. Bell, J. E. Bercaw, C. Creutz, E. Dinjus, D. A. Dixon, K. Domen, D. L. DuBois, C. A. Eckert, E. Fujita, D. H. Gibson, W. A. Goddard, D. W. Goodman, J. Keller, G. J. Kubas, H. H. Kung, J. E. Lyons, L. E. Manzer, T. J. Marks, K. Morokuma, K. M. Nicholas, R. Periana, L. Que, J. Rostrup-Nielson, W. M. H. Sachtler, L. D. Schmidt, A. Sen, G. A. Somorjai, P. C. Stair, B. R. Stults and W. Tumas, Chem. Rev., 2001, 101, 953–996 CrossRef CAS PubMed.
- M. Aresta, Carbon Dioxide as Chemical Feedstock, John Wiley & Sons, 2010 Search PubMed.
- B. Schäffner, F. Schäffner, S. P. Verevkin and A. Börner, Chem. Rev., 2010, 110, 4554–4581 CrossRef PubMed.
- T. Sakakura, J. C. Choi and H. Yasuda, Chem. Rev., 2007, 107, 2365–2387 CrossRef CAS PubMed.
- M. Aresta and A. Dibenedetto, Dalton Trans., 2007, 2975–2992 RSC.
- M. North, R. Pasquale and C. Young, Green Chem., 2010, 12, 1514–1539 RSC.
- M. Yoshida and M. Ihara, Chem.–Eur. J., 2004, 10, 2886–2893 CrossRef CAS PubMed.
- T. Yano, H. Matsui, T. Koike, H. Ishiguro, H. Fujihara, M. Yoshihara and T. Maeshima, Chem. Commun., 1997, 1129–1130 RSC.
- K. Yamaguchi, K. Ebitani, T. Yoshida, H. Yoshida and K. Kaneda, J. Am. Chem. Soc., 1999, 121, 4526–4527 CrossRef CAS.
- N. Kihara, N. Hara and T. Endo, J. Org. Chem., 1993, 58, 6198–6202 CrossRef CAS.
- L. P. Guo, C. M. Wang, X. Y. Luo, G. K. Cui and H. R. Li, Chem. Commun., 2010, 46, 5960–5962 RSC.
- V. Calo, A. Nacci, A. Monopoli and A. Fanizzi, Org. Lett., 2002, 4, 2561–2563 CrossRef CAS PubMed.
- B. R. Buckley, A. P. Patel and K. G. Wijayantha, Chem. Commun., 2011, 47, 11888–11890 RSC.
- L. N. Han, S. W. Park and D. W. Park, Energy Environ. Sci., 2009, 2, 1286–1292 CAS.
- H. S. Kim, J. J. Kim, H. Kim and H. G. Jang, J. Catal., 2003, 220, 44–46 CrossRef CAS.
- J. M. Sun, S. I. Fujita, F. Y. Zhao and M. Arai, Green Chem., 2004, 6, 613–616 RSC.
- H. C. Cho, H. S. Lee, J. Chun, S. M. Lee, H. J. Kim and S. U. Son, Chem. Commun., 2011, 47, 917–919 RSC.
- J. Melendez and M. North, Chem. Commun., 2009, 2577–2579 RSC.
- A. Buchard, M. R. Kember, K. G. Sandeman and C. K. Williams, Chem. Commun., 2011, 47, 212–214 RSC.
- A. Decortes, M. M. Belmonte, J. Benet-Buchholz and A. W. Kleij, Chem. Commun., 2010, 46, 4580–4582 RSC.
- H. S. Kim, J. J. Kim, H. N. Kwon, M. J. Chung, B. G. Lee and H. G. Jang, J. Catal., 2002, 205, 226–229 CrossRef CAS.
- H. Zhou, W. Z. Zhang, C. H. Liu, J. P. Qu and X. B. Lu, J. Org. Chem., 2008, 73, 8039–8044 CrossRef CAS PubMed.
- J. Sun, S. J. Zhang, W. G. Cheng and J. Y. Ren, Tetrahedron Lett., 2008, 49, 3588–3591 CrossRef CAS.
- J. M. Sun, S. I. Fujita, F. Y. Zhao and M. Arai, Appl. Catal., A, 2005, 287, 221–226 CrossRef CAS.
- L. N. He, H. Yasuda and T. Sakakura, Green Chem., 2003, 5, 92–94 RSC.
- S. S. Wu, X. W. Zhang, W. L. Dai, S. F. Yin, W. S. Li, Y. Q. Ren and C. T. Au, Appl. Catal., A, 2008, 341, 106–111 CrossRef CAS.
- J. J. Peng and Y. Q. Deng, New J. Chem., 2001, 25, 639–641 RSC.
- J. Sun, L. J. Han, W. G. Cheng, J. Q. Wang, X. P. Zhang and S. J. Zhang, ChemSusChem., 2011, 4, 502–507 CrossRef CAS PubMed.
- J. K. Lee, Y. J. Kim, Y. S. Choi, H. Lee, J. S. Lee, J. Hong, E. K. Jeong, H. S. Kim and M. Cheong, Appl. Catal., B, 2012, 111–112, 621–627 CrossRef CAS.
- H. Sun and D. J. Zhang, J. Phys. Chem. A, 2007, 111, 8036–8043 CrossRef CAS PubMed.
- J. Q. Wang, J. Sun, W. G. Cheng, K. Dong, X. P. Zhang and S. J. Zhang, Phys. Chem. Chem. Phys., 2012, 14, 11021–11026 RSC.
- J. Q. Wang, K. Dong, W. G. Cheng, J. Sun and S. J. Zhang, Catal. Sci. Technol., 2012, 2, 1480–1484 CAS.
- Y. Ren, C. H. Guo, J. F. Jia and H. S. Wu, J. Phys. Chem. A, 2011, 115, 2258–2267 CrossRef CAS PubMed.
- S. Foltran, R. Mereau and T. Tassaing, Catal. Sci. Technol., 2014, 4, 1585–1597 CAS.
- J. Ma, J. Liu, Z. Zhang and B. Han, Green Chem., 2012, 14, 2410–2420 RSC.
- C. J. Whiteoak, A. Nova, F. Maseras and A. W. Kleij, ChemSuschem, 2012, 5, 2032–2038 CrossRef CAS PubMed.
- F. Castro-Gómez, G. Salassa, A. W. Kleij and C. Bo, Chem.–Eur. J., 2013, 19, 6289–6298 CrossRef PubMed.
- Z. Zhang, L. Xu and W. Feng, RSC Adv., 2015, 5, 12382–12386 RSC.
- M. Alves, R. Mereau, B. Grignard, C. Detrembleur, C. Jerome and T. Tassaing, RSC Adv., 2016, 6, 36327–36335 RSC.
- L. Wang, T. Huang, C. Chen, J. Zhang, H. He and S. Zhang, J. CO2 Util., 2016, 14, 61–66 CrossRef CAS.
- L. Wang, X. F. Jin, P. Li, J. L. Zhang, H. Y. He and S. J. Zhang, Ind. Eng. Chem. Res., 2014, 53, 8426–8435 CrossRef CAS.
- W. L. Dai, B. Jin, S. L. Luo, X. B. Luo, X. M. Tu and C. T. Au, J. Mol. Catal. A: Chem., 2013, 378, 326–332 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox. Gaussian 09, Revision A.02, Gaussian Inc. Wallingford, CT, 2009 Search PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- M. D. Ganji, S. M. Hosseini-Khah and Z. Amini-Tabar, Phys. Chem. Chem. Phys., 2015, 17, 2504–2511 RSC.
- M. Piccardo, E. Penocchio, C. Puzzarini, M. Biczysko and V. Barone, J. Phys. Chem. A, 2015, 119, 2058–2082 CrossRef CAS PubMed.
- P. M. W. Gill, B. G. Johnson, J. A. Pople and M. J. Frisch, Chem. Phys. Lett., 1992, 197, 499–505 CrossRef CAS.
- K. Fukui, Acc. Chem. Res., 1981, 14, 363–368 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- A. E. Reed, R. B. Weinstock and F. Weinhold, J. Chem. Phys., 1985, 83, 735–746 CrossRef CAS.
- A. E. Reed, L. A. Curtiss and F. Weinhold, Chem. Rev., 1988, 88, 899–926 CrossRef CAS.
- C. H. Guo, X. M. Zhang, J. F. Jia and H. S. Wu, J. Mol. Struct.: THEOCHEM, 2009, 916, 125–134 CrossRef CAS.
- C. G. Fernando, S. Giovanni, W. K. Arjan and B. Carles, Chem.–Eur. J., 2013, 19, 6289–6298 CrossRef PubMed.
- C. Wu, T. P. Senftle and W. F. Schneider, Phys. Chem. Chem. Phys., 2012, 14, 13163–13170 RSC.
- J. Z. Zhang, J. Cai, H. F. Dong, J. Q. Wang, X. P. Zhang and S. J. Zhang, Ind. Eng. Chem. Res., 2013, 52, 5835–5841 CrossRef CAS.
- H. B. Xie, Y. Z. Zhou, Y. K. Zhang and J. K. Johnson, J. Phys. Chem. A, 2010, 114, 11844–11852 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra20174a |
| This journal is © The Royal Society of Chemistry 2016 |
