Preparation of a liquefied banana pseudo-stem based PVAc-nanosilica hybrid membrane and its modification by octadecyltrichlorosilane†
Abstract
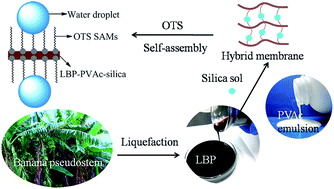
A novel addition cured liquefied banana pseudo-stem based PVAc (LBP–PVAc) composite membrane with a nano-silica sol was successfully prepared in this study. Surface hydrophobic modification has been carried out using a octadecyltrichlorosilane (OTS) reagent. Scanning electron microscopy (SEM) results showed that nano-silica particles distribute well in the LBP–PVAc system and the structural homogeneity of the hybrid membrane is enhanced. Fourier transform infrared spectroscopy (FTIR) confirmed the self-assembled monolayers of octadecyltrichlorosilane (OTS SAMs) formed on the surface of membranes. Anti-moisture absorption is highly enhanced as water contact angle (CA) on the modified membrane is about 134.88° and relatively stable. The optimal mechanical tensile strength and break elongation of the hybrid membrane were increased by 425.79% and 250.81%, when the concentration of nano-silica is 0.5 wt%. Thermogravimetric (TG) analysis suggested that the thermal stability of the cured hybrid membranes is also improved.

 Please wait while we load your content...
Please wait while we load your content...