Multi-modal MR imaging and magnetic hyperthermia study of Gd doped Fe3O4 nanoparticles for integrative cancer therapy†
Nanasaheb D. Thorat *ab,
Raghvendra A. Boharac,
Hemraj M. Yadavd and
Syed A. M. Tofailab
*ab,
Raghvendra A. Boharac,
Hemraj M. Yadavd and
Syed A. M. Tofailab
aDepartment of Physics, Bernal Institute, University of Limerick, Limerick, Ireland. E-mail: nanasaheb.thorat@ul.ie; thoratnd@gmail.com
bMaterial and Surface Science Institute, Bernal Institute, University of Limerick, Limerick, Ireland
cResearch and Innovations for Comprehensive Health Care (RICH), Dr D. Y. Patil Hospital and Research Center, D. Y. Patil University, Kolhapur, India
dDepartment of Materials Science & Engineering, University of Seoul, 02504, South Korea
First published on 29th September 2016
Abstract
Among different kinds of cancer theranostic mediators, gadolinium (Gd) doped iron oxide nanoparticles are one of the most promising candidates in combining diagnostics (imaging) and therapeutics (molecular therapy) functions in a single, multimodal platform. Due to its larger size, the doping of Gd into the Fe3O4 is difficult. We have overcome this difficulty by modifying a polyol based reflux method that has been previously used for, for example, cobalt–zinc (Co–Zn) doping of ferrites but not for doping with Gd. This modified approach allowed a facile synthesis of Gd-doped superparamagnetic iron oxide (Fe3O4) nanoparticles (GdSPIONPs) with a lower Curie temperature (Tc) for hyperthermia superparamagnetism with low coercivity, both T1 and T2 based MRI contrast enhancements, low cytotoxicity and optimal hemocompatibility. Such a combination of theranostics properties in a single nanosystem is unprecedented and highly desirable for heat controlled magnetic hyperthermia in minimizing treatment resistance, and maximizing treatment efficacy.
1. Introduction
Magnetic nanomaterials have great potential as mediators for nano-biomedicine especially with promising success demonstrated in cancer theranostics (i.e. a combination of therapy and diagnosis).1–3 Novel magnetic nanoparticles (MNPs) such as superparamagnetic iron oxide nanoparticles (SPIONPs) could enable better cancer theranostics by combining magnetic fluid hyperthermia (MFH) treatment with magnetic resonance imaging (MRI).4,5 SPIONPs based multimodal cancer therapy thus integrates diagnosis and monitoring cancer using imaging modalities such as MRI with concurrent MFH thermotherapy.6–9 T1 − T2 weighted dual modal MRI scans of tumors or tumorous tissues can provide more detailed pathology information in the diagnosis and monitoring of cancer treatment. Dual model imaging can clear illustration of tumor barriers, which allow for authentic understanding of tumor distribution and its response to adjuvant therapies such as MFH thermotherapy.10,11Even though SPIONPs were specifically developed for use as T2-weighted MR imaging T2-weighted darker contrast signals acquired with SPIONPs becomes readily disrupted due to artifacts from metal deposits, blood coagulation and excessive bleeding.12,13 This calls for the development of T1 − T2 dual-modal imaging contrast agents for achieving more authentic, artifact-free diagnosis. Gadolinium-chelated diethylenetriaminopentaacetic acid (Gd-DTPA) is currently one of the most clinically used T1 contrast agent, which is a small and non-targeted compound. Sometimes Gd-DTPA disperse into the interstitials of tissues and organs and results in poor signal enhancement.14–16 The incorporation of, for example, body temperature-paramagnetic lanthanides into the SPIONPs can enable complementary imaging modalities such as T1 weighted MR imaging.
In using thermal treatments such as hyperthermia for cancer therapies, a localized temperature application is required because otherwise systemic changes in body temperature would be uncomfortable and potentially damaging. SPIONPs have the potential to provide highly localized heat directly to the area of treatment while also providing clinicians with an accurate picture of particle localization, concentration and therapy monitoring.17 Despite preliminary successes, the great potential of SPIONPs has been circumvent by factors such as the need for high magnetic field strengths for therapeutically-relevant heating.18 On the other hand clinical applications of these nanoparticles as T2 contrast agents (CAs) are still quite limited by the inherent darkening contrast effect. Such limitations can be overcome by the introduction of appropriate lanthanide ions e.g. gadolinium (Gd) in the lattice of IONPs.19 Currently, paramagnetic gadolinium (Gd)-based chelates are used as preferred agents for T1-contrast enhancement because lanthanide ions possess more unpaired electrons in their f-electronic orbital. Gd chelates, however, suffer from a short body circulation time due to their low molecular weights and potential in vivo toxicity.
Another important parameter is the development of SPIONPs as MFH mediators that can raise temperature within a range of between 315 and 319 K during the in vivo cancer therapy. Current MFH utilizes colloid-stabilized magnetic fluids based on SPIONPs with a Curie temperature (Tc) of ∼858.15 K.20 These SPIONPs will continue to generate heat unless it reaches this Curie temperature, which is very high. The Tc of magnetic materials should be lowered to fall within the 315–319 K range to prevent from overheating.21,22 This lowering of the Tc of SPIONPs have been attempted by doping alkaline earth and transition metals such as Mg, Mn, Co.23 Inner transition metal ions, especially Gd3+, have been developed as a new generation of T1 contrast agents which offer many unique advantages.14 The doping of Gd into the Fe3O4 is not trivial as size of Gd ion is quite large to be incorporated into the inverse spinel structure of Fe3O4. For this purpose, we have used a polyol based reflux method that has previously been used for both metal-doped and pristine iron oxide nanoparticles (IONPs) synthesis but not for doping with Gd.24
This modified approach allowed a facile synthesis of Gd-doped superparamagnetic iron oxide (Fe3O4) nanoparticles (GdSPIONPs) with a lower Tc for hyperthermia and MRI contrast enhancement. We have optimized the doping by varying the amount of Gd3+ ions into magnetite. We report the structural, spectroscopic (photoelectron and Mössbauer), magnetic and biocompatibility properties of these GdSPIONPs for hyperthermia and the T1 and T2 relaxometric properties for MRI contrast improvement. These GdSPIONPs possess lower Curie temperature, superparamagnetism with low coercivity, and both T1 and T2 based MRI contrasts. Such an unprecedented combination of desired theranostics properties in a single nanosystem is further associated with a high Specific Absorption Rate (SAR) of magnetic radiation by GdSPIONPs. Together, these novel GdSPIONPs can become strong candidates for multimodal integrated cancer theranostics especially for heat controlled magnetic hyperthermia by minimizing treatment resistance, and maximizing treatment efficacy.
2. Experimental
2.1 Synthesis of Gd-doped superparamagnetic iron oxide nanoparticles (GdSPIONPs)
A stoichiometric series of four samples was prepared with the general formula GdxFe3−xO4 (x = 0.02, 0.04, 0.06 and 0.08 mol%) by a modified high temperature decomposition reflux method previously applied for co-doping Co and Zn in ferrites. Salts of FeCl2, FeCl3 and GdCl3 were dissolved in desired stoichiometric proportions in diethylene glycol (40 mL) and heated at 383 K in nitrogen umbrella under vigorous magnetic stirring for 2 h. The reaction mixture was then refluxed at 433 K for another 6 h, during reflux fine black colored colloidal nanoparticles formed in the reaction mixture. The mixture was cooled to room temperature, and particles were washed with ethanol and hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) for several times and separated by centrifugation. Finally, these separated particles were dried in vacuum for 12 h. The series of prepared sample were labelled as GdF2 (Gd0.02Fe2.98O4), GdF4 (Gd0.04Fe2.96O4), GdF6 (Gd0.06Fe2.94O4) and GdF8 (Gd0.08Fe2.92O4).
5) for several times and separated by centrifugation. Finally, these separated particles were dried in vacuum for 12 h. The series of prepared sample were labelled as GdF2 (Gd0.02Fe2.98O4), GdF4 (Gd0.04Fe2.96O4), GdF6 (Gd0.06Fe2.94O4) and GdF8 (Gd0.08Fe2.92O4).
2.2 Characterization
X-ray diffraction (XRD) phase analysis on a Philips PW-3710 diffractometer using Cu-Kα radiation in the 2θ range 20 to 80°. XRD patterns were analyzed by X'pert high score software program. The particle sizes and shape of the nanoparticles was determined by transmission electron microscopy (TEM, Philips CM200 model, operating voltage 20–200 kV, resolution 2.4 Å). X-ray photoelectron spectroscopy was conducted using a Physical Electronics 5600 Multi-technique System with monochromatic Al Ka radiation. The photoluminescence (PL) spectra of the representative samples were recorded by using a JASCO FP-750. The room temperature Mössbauer spectra of the samples were recorded using a Mössbauer spectrometer [Mössbauer spectrometer system (Type: MC1002), Nucleonix Systems Pvt. Ltd., Hyderabad, India]. The source employed was Co-57 in Rh matrix of strength 50 mCi. The calibration of the velocity scale was done by using a α-Fe metal foil. The outer line width of calibration spectra is 0.29 mm s−1. c spectra were fitted by Win Normos program assuming Lorentzian line shapes.Magnetization measurements including saturation magnetization, zero-field cooling (ZFC) and field cooling (FC) measurements were performed on a Quantum Design SQUID magnetometer. FC-ZFC measurements were taken in the range 5 to 400 K at an applied magnetic field of 500 Oe. Field dependent hysteresis loops of magnetization (M–H) was measured at two different temperatures namely 5 and 300 K with an applied field range from 0 to ±2 × 104 Oe (2 tesla). Magnetic fluid hyperthermia (MFH) was performed in accordance with protocol and reported in our recent publication.25,26 T1 and T2 relaxation times of all samples were measured for various concentrations of NPs by using a 3 T clinical MRI scanner (General Electric Healthcare, USA). Samples with different concentrations of NPs were prepared by dilution with Milli-Q water. T1 and T2-weighted images were obtained with the following parameters: the repetition time, TR, was 5000 ms, and the echo times were 30, 40, 60, 80, 100, 150, and 200 ms. Relaxation rates (1/T1 and 1/T2) were measured and plotted against the concentrations of NPs. The relaxivities r1 and r2 were then obtained from the slopes of these curves. The T1 and T2 relaxation times for each concentration were estimated by fitting the decay curve with use of the exponential relation I(TE) = Ioe(−TE/T2), where TE is the echo time and I(TE) is the MRI signal intensity at each TE. The r2 relaxation values (mM−1 s−1) were calculated from the slope of the linear plots of 1/T2 against the NP concentration. The comparative in vitro cytotoxicity study of GdSPIONPs was performed on L929 and MCF7 cells obtained from the National Centre for Cell Sciences, Pune (India) by MTT assay.27 Hemolysis activity of GdSPIONPs is assessed and pre-treatment was conducted to obtain HRBCs for hemolysis assay according to the literature, human blood samples are collected from D. Y. Patil Hospital, Kolhapur, India.28,29
3. Result and discussions
3.1 Structural and magnetic study
The synthesis of monodisperse GdSPIONPs with nano-sizes was performed by polyol reflux method. FeCl3, FeCl2 and GdCl3 precursors dissolved in diethylene glycol (DEG) used to produce GdSPIONPs with diameters of 10 nm were obtained in the reflux time of 6 h. For comparison, we also obtained monodisperse pristine Fe3O4 with a diameter of 10 nm using the same synthesis procedure but without using any Gd precursor. DEG was used both as a solvent and a reducing agent, which plays an important role in the formation of the spinel phase. As a good capping agent with two hydroxyl groups, DEG can hold free metal ions tightly in the solution. In the modified method successful Gd doping was achieved by resorting to a higher temperature and longer duration of DEG mediated metal salt conversion in acetates.XRD was used to study the crystallinity and structural properties of the GdSPIONPs. As shown in Fig. 1a, the X-ray powder diffraction patterns of GdF2–GdF8 samples confirmed the formation of a single phase of the Gd-doped magnetite, which had the cubic spinel structure of the well-studied Fe3O4. There was no significant change in the X-ray powder pattern of the GdSPIONPs compared to the parent compound, Fe3O4 and no peak shift was observed in the XRD patterns of Gd-doped samples. GdSPIONPs showed a cubic unit cell with lattice parameter, a of 0.8391, 0.8399, 0.8421 and 0.8446 nm for GdF2, GdF4, GdF6 and GdF8, respectively. These values are, expectedly, slightly higher than that of the pristine Fe3O4 with a = 0.8373 nm (space group Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, a = 8.384 Å).30 Lattice constants increased with increasing Gd concentration and this is because of the larger ionic radius of Gd3+ (0.0938 nm) compared to that of Fe3+ (0.067 nm).31 Gd3+ ions are expected to occupy the octahedral (B) sites of spinel structure in place of Fe3+ ions, and result in an internal strain to make the lattice distorted with the expansion of the unit cell.32 The average particles size of the GdSPIONPs was estimated by using the Debye–Scherrer model for the (311) reflection in the powder pattern shown in Fig. 1a. The calculated average of the crystallite size was 5.0, 5.6, 5.8, 6.1 and 6.2 nm for Fe3O4, GdF2, GdF4, GdF6 and GdF8, respectively. This indicates that Gd3+ doping has slightly improved the crystallite size but did not significantly change the crystallinity of magnetite as it has been found in previous reports.33
m, a = 8.384 Å).30 Lattice constants increased with increasing Gd concentration and this is because of the larger ionic radius of Gd3+ (0.0938 nm) compared to that of Fe3+ (0.067 nm).31 Gd3+ ions are expected to occupy the octahedral (B) sites of spinel structure in place of Fe3+ ions, and result in an internal strain to make the lattice distorted with the expansion of the unit cell.32 The average particles size of the GdSPIONPs was estimated by using the Debye–Scherrer model for the (311) reflection in the powder pattern shown in Fig. 1a. The calculated average of the crystallite size was 5.0, 5.6, 5.8, 6.1 and 6.2 nm for Fe3O4, GdF2, GdF4, GdF6 and GdF8, respectively. This indicates that Gd3+ doping has slightly improved the crystallite size but did not significantly change the crystallinity of magnetite as it has been found in previous reports.33
The successful Gd doping in magnetite and chemical composition of GdSPIONPs was additionally investigated by XPS. Fig. 1b shows the survey spectrum of as-prepared GdF2 sample. From XPS, the typical binding energies for the characteristic peaks of O 1s, Fe 2p and Gd 3d were confirms the presence of oxygen, gadolinium and iron elements in the GdSPIONPs. The bonding energies at 711.4 eV and 724.6 eV were assigned to Fe 2p3/2 and Fe 2p1/2 cations, respectively; confirmed the presence of mixed Fe(III) and Fe(II). The existence of Gd(III) was confirmed by the Gd 4p peaks at 142.6 and 157.9 eV, while the oxygen peak was observed at 527 eV.
The particle size of GdSPIONPs was determined by TEM. Fig. S1a and b† represents TEM image of GdF2 and HRTEM images of GdF2, GdF4, GdF6 and GdF8, respectively. From the TEM image of GdF2 (Fig. S1a†), it can be seen that the GdSPIONPs were composed of tiny nanocrystals with sizes ranging from 10 to 15 nm. Further, the effect of Gd doping in Fe3O4 was identified by HRTEM analysis. As shown in Fig. S1b,† the distance between two adjacent planes of GdF2, GdF4, GdF6 and GdF8 nanocrystal was measured to be 0.341, 0.346, 0.349 and 0.351 nm, respectively, corresponding to the (311) plane. The distance between two adjacent planes of Fe3O4 is 0.319 nm (not shown in the figure) and after Gd ions doping into crystal lattice of Fe3O4, the distance between these adjacent planes increased gradually with increase in Gd concentration, results are comparable to recent reports.14
Mössbauer spectroscopy is an excellent tool for probing the local environment of Fe atoms present in such a complex matrix. Fig. 2 shows the room temperature Mössbauer spectra of Fe3O4, GdF2 and GdF8 samples, and the respective hyperfine parameters are listed in Table S1.† Two sets of six-line hyperfine patterns are observed in all samples, indicating the presence of Fe in both A and B sites.34 The Mössbauer spectrum of pure Fe3O4 and Gd samples consist of two sextets, one corresponding to tetrahedral sites (A site) high-spin Fe3+ and the other corresponding to octahedral sites i.e. Fe2+ (B site).35 The recognition of A and B sites can be made from isomer-shift data, hyperfine distribution width and integrated intensity. An increase in the Gd concentration has led to a decrease in the hyperfine field strength at A and B sites at unequal rates. The Gd substituting Fe results in a reduction of the strength and an increase in the distribution magnetic hyperfine field. The B site hyperfine field and distribution are relatively less affected, however, and confirms a hypothesis that the Gd generally substitutes Fe at A sites.36
Temperature dependent Mössbauer spectroscopy measurements should provide further insights in to the effect of Gd-doping especially in reducing the Curie temperature. Both temperature and field variant magnetic properties were determined using a SQUID-VSM. Fig. S2† shows the variation of magnetization M as a function of temperature (T) of all GdSPIONPs samples in the range 5 to 350 K in an applied magnetic field of 500 Oe recorded in ZFC and FC mode. All ZFC and FC curves shows near-coincidence at very low temperatures. This is one of the characteristic features of a superparamagnetic system. Fig. S2† also shows that all GdSPIONPs samples studied are superparamagnetic. The Curie transition temperature, Tc, of these samples were determined from the maxima of the dM/dT versus T curve.37 The Tc was found to be 350, 372, 389 and 400 K for GdF2, GdF4, GdF6 and GdF8 samples, respectively. This is significantly lower than pristine Fe3O4 (∼750 K) of equivalent size.38 This lowering of Tc is remarkable as it means that our GdSPIONPs are capable of generating heat that keeps the temperature rise due to hyperthermia within a narrow, desirable range rather than leading to overheating.
Fig. 3 shows the M–H curves of GdSPIONPs at 300 and 5 K, respectively. All samples exhibited superparamagnetic behaviors with almost zero coercivity at 300 K. At this temperature, net magnetization (Ms) values were found to be 73.57, 66.79, 62.21, 58.60 and 52.23 emu g−1 for Fe3O4, GdF2, GdF4, GdF6 and GdF8, respectively. The decrease in the magnetization with increasing Gd content in Fe3O4 can be attributed to the disruption caused by Gd doping in the long-range order of magnetic spins in Fe3O4.33 The superparamagnetism observed in all samples ensures that under an external magnetic field the spin order of Gd(III) in the inner location of GdSPIONPs should have the same direction.14 Despite the decrease in the saturation magnetization (Ms) upon Gd doping, all GdSPIONPs would meet the requirements for using as an MFH mediator along with positive and negative MRI contrast agent. More significantly, all of the synthesized GdSPIONPs show a great potential in Tc-controlled magnetic hyperthermia along with dual mode T1 and T2 contrast imaging and relaxation enhancements. These NPs showed an excellent combination of Gd-doping, superparamagnetism, efficient net magnetization and, above all and more importantly, low Tc that accomplish prime requirements of MFH.
3.2 Hyperthermia properties of GdSPIONPs
Magnetic field-dependent temperature kinetics and specific absorption rates (SAR) of GdSPIONPs in water were studied to investigate their hyperthermia properties. Mössbauer and magnetic measurements discussed in the previous section showed that GdSPIONPs synthesized are superparamagnetic. The heat generated by GdSPIONPs is attributed to power losses accomplished by Brownian or Néel's spin relaxations described by:| P = μ0πχ′′fH2 | (1) |
 | (2) |
 | (3) |
In addition to superparamagnetic relaxation losses, particles showing a hysteresis behavior would also contribute heat generation due to magnetic hysteresis. The amount of heat (A) generated or released by MNPs during one cycle of the magnetic field equals to the area within the hysteresis loop and given by the following relation
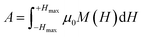 | (4) |
Then the SAR is estimated as:
| SAR = Af | (5) |
Fig. 4 represents temperature kinetics, temporal development and SAR of GdSPIONPs in aqueous solution (1 mg mL−1) at various AC magnetic field amplitudes. Fig. 4a shows the temperature kinetics of all GdSPIONPs samples at field amplitude of 500 Oe for 10 min. Overall, temperature increased with the heating time for all samples. Fig. 4b quantitatively compare the maximum temperature (within 10 min) reached by Fe3O4 and the all other GdSPIONPs samples at the same concentration.
All GdSPIONPs samples and the pristine Fe3O4 show comparable temperature development linearly with an increase in the bearing field. Compared to Fe3O4, the GdSPIONPs, however, entailed a lower threshold for temperature increase after 10 minutes of heating. The Fe3O4 sample attain the maximum temperature of 327 K at 500 Oe while the increase in GdSPIONPs is relatively lower and within the desirable range for MFH. This is highly encouraging data especially for expanding investigation with these GdSPIONPs for temperature controlled in vitro and in vivo MFH.
The hyperthermal efficiency of GdSPIONPs expressed as SAR in W g−1 has been quantitatively compared in Fig. 4c. The temperature became saturated for each GdSPIONPs at higher magnetic field amplitudes. Both SAR and the maximum temperature achieved within 10 min presented a linear relationship with the applied AC magnetic field amplitude. SAR values of GdSPIONPs samples at 500 Oe applied filed are 300.24, 266.1, 252.83, 189.21 W g−1 for GdF2, GdF4, GdF6 and GdF8, respectively. These values are slightly lower than that of the Fe3O4 (341.09 W g−1) and much higher than that reported for chitosan-coated MnFe2O4 (22.35 W g−1).39 An elevated temperature rise was, however, observed for only Fe3O4 when compared to the GdSPIONPs. The reduction in temperature rise in GdSPIONPs can be attributed to the overall decrease in the net magnetization due to Gd content. The net magnetization of NPs is directly related to temperature rise and crucial factor for their hyperthermia response.40–42 However, hyperthermia properties are affected by not only magnetization but also by particle size, magnetic anisotropy, colloidal stability and hysteresis behavior.43,44 An in-depth investigation to find the detailed mechanism behind the hyperthermia response GdSPIONPs is needed.
3.3 MRI study
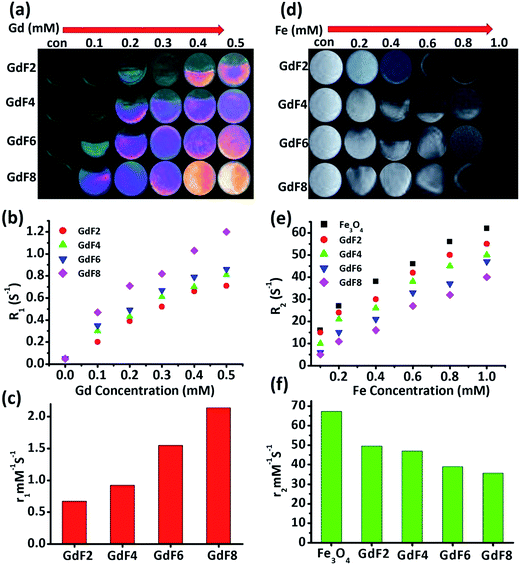
The feasibility of GdSPIONPs as a T1 − T2 dual-modal MRI contrast agent is investigated by using a 3T clinical MRI scanner. The longitudinal relaxivity (r1) and transverse relaxivity (r2) were determined by plotting the inverse relaxation time against the Gd and Fe concentration, respectively. Molar concentrations of Fe and Gd elements in GdSPIONPs were estimated using inductively coupled plasma mass spectrometry (ICP-MS). For transverse relaxivity, pristine Fe3O4 prepared by similar method was set as the control. T1-Weighted color phantom images for GdSPIONPs at different Gd concentrations (0.1 to 0.5 mM) are shown in the Fig. 5a. The T1 signal became brighter when the concentration of GdSPIONPs was increased. With increasing molar concentration of Gd, we observe an increase in the T1 contrast and longitudinal relaxivity values (Fig. 5b and c). The GdSPIONPs exhibited r1 values of 0.65, 0.91, 1.55 and 2.11 mM−1 s−1 for GdF2, GdF4, GdF6 and GdF8, respectively. The r1 values are much higher than those obtained using the same concentration of conventional gadolinium oxide and Gd-DTPA NPs.45T2-Weighted images for GdSPIONPs with respect to Fe concentrations (0.2–1 mM) are shown in the Fig. 5d. In a representative T2 weighted image, the T2 MR contrast became darker within increasing GdSPIONPs concentration. The 1/T2 i.e. inverse relaxation time, collected at a steady frequency of 35 MHz, was plotted with respect to Fe concentration (Fig. 5e). r2 value of GdSPIONPs samples are calculated to be 49.12, 46.76, 38.31 and 34.98 mM−1 for GdF2, GdF4, GdF6 and GdF8, respectively. However, Fe3O4 has shown a value of 67.23 mM−1 s−1 (Fig. 5f) implying that the effect of Gd-doping on the r2 value is minor. The MRI results thus suggest that GdSPIONPs can be used as negative and positive contrast agents by a collectively enhanced contrast and an increase in r1 and r2 relaxivity.
3.4 Biocompatibility study
In vitro toxicity assessment was performed to evaluate the biocompatibility of GdSPIONPs. To evaluate explicit cytotoxicity/biocompatibility of the GdSPIONPs, we tested cytotoxicity on different cells by varying incubation time and dose of NPs.Fig. 6a shows the cytotoxicity profile of GdSPIONPs (1 mg mL−1) on L929 and MCF7 cells after 24 h of incubation. A negligible cellular toxicity was found for GdSPIONPs even when Gd was doped at higher concentration. A slight difference can be observed on the cell viability with different cell lines. The difference in cell viability on various cell lines are likely to occur due to individual cell types and surface property, cellular morphology, and differential cell division.46 Furthermore, different cell types show different metabolic activity.47 The results obtained were systematic, and no acute or intense changes has been observed when the amount of Gd changed. The cell viability of bare Fe3O4 on L929 and MCF7 cells are 81.17 and 79.86, respectively. The cell viability of GdF2, GdF4, GdF6 and GdF8 samples in case of higher concentration (1 mg mL−1) by MTT assay for 24 h incubation on L929 cells was calculated to be 80.96, 78.54, 76.12 and 73.04%, respectively. The cell viability of corresponding samples on MCF7 cells is 78.75, 78.01, 74.14 and 72.59%.
 | ||
| Fig. 6 Comparative data of the (a) cytotoxicity and (b) hemolysis activity of GdF2, GdF4, GdF6 and GdF8 samples with respect to Fe3O4. | ||
Materials used for in vivo applications are generally transferred through blood. The hemocompatibility of GdSPIONPs would need to be addressed before any in vivo trial. Lanthanide elements such as La, Gd have been reported as hemolytic elements and generate pores into the membrane of HRBCs.48 Fig. 6b shows the GdSPIONPs hemolytic behavior at concentration 1 mg mL−1 with various time intervals, control of Fe3O4 sample is also studied. At such dosage for up to a 24 h period, there was no significant quantitative induction of hemolytic activity by any of these GdSPIONPs (Fig. 6b). The induced hemolysis by GdF2, GdF4, GdF6 and GdF8 samples was 8.44, 9.60, 11.51 and 12.14% for 24 h. The control Fe3O4 sample shows 7.88% hemolysis for 24 h. The hemolysis observed for GdSPIONPs with such a concentration (1 mg mL−1 for 24 h) lies near the prescribed permissible limit as per ASTMF-756-08.49 The negligible hemolytic activity of GdSPIONPs in a higher concentration range along with their excellent biocompatibility and would facilitate their use excellence in MFH and MRI based combined cancer theranostics.
4. Conclusion
This article reports the successful doping of Fe3O4 nanoparticles by Gd using a slightly modified polyol based synthesis method. We found that a single nanosystem such as the Gd-doped superparamagnetic nanoparticles can possess a combination of many desirable properties for magnetic fluid hyperthermia therapy using MRI. These properties include a significantly low Curie temperature (Tc), superparamagnetic T1 − T2 dual-model MR imaging with high contrast and magnetic hyperthermia within a narrow temperature window in the range of 315–319 K. The facile method reported here for the synthesis of highly magnetic and size controlled GdSPIONPs has the ability of incorporating a large portion of gadolinium inside a magnetite lattice, which allows an unprecedented combination of simultaneous magnetic hyperthermia and superparamagnetic T1 − T2 dual-model MR imaging.Considering therapeutic efficacy of only magnetic hyperthermia which is limited by various factors discussed in the article, we believe that our proposed GdSPIONPs can overtake physical and biological drawbacks of magnetic hyperthermia and provide high therapeutic efficacy. Furthermore, this system can be applicable to conjugate chemotherapeutic drugs for intracellular drug release and facilitate the detection of the cancer cells after hyperthermia using T1 − T2 dual-model MR imaging. The elementary concept promoted in this study should be easily applicable to an extensive non-conventional and non-conventional cancer therapy especially for heat controlled magnetic hyperthermia by minimizing treatment resistance and maximizing treatment efficacy.
Acknowledgements
Authors are thankful to Dr Raghumani S. Ningthoujam from chemistry division BARC India for Mossbauer measurements and Dr Sawanta Mali, Chonnam National University, South Korea for XPS measurements. The work is financially supported by the Irish Research Council Government of Ireland Postdoctoral Fellowship-2015, Grant No. GOIPD/2015/320.References
- O. L. Gobbo, K. Sjaastad, M. W. Radomski, Y. Volkov and A. Prina-Mello, Theranostics, 2015, 5, 1249–1263 CrossRef CAS PubMed.
- H. Arami, A. Khandhar, D. Liggitt and K. M. Krishnan, Chem. Soc. Rev., 2015, 44, 8576–8607 RSC.
- A. Ray Chowdhuri, D. Bhattacharya and S. K. Sahu, Dalton Trans., 2016, 45, 2963–2973 RSC.
- Y. Zhou, X. Liang and Z. Dai, Nanoscale, 2016, 8, 12394–12405 RSC.
- R. A. Revia and M. Zhang, Mater. Today, 2015, 19, 157–168 CrossRef PubMed.
- G. Hemery, E. Garanger, S. Lecommandoux, A. D. Wong, E. R. Gillies, B. Pedrono, T. Bayle, D. Jacob and O. Sandre, J. Phys. D: Appl. Phys., 2015, 48, 494001 CrossRef.
- I. Conde-Leboran, D. Baldomir, C. Martinez-Boubeta, O. Chubykalo-Fesenko, M. del Puerto Morales, G. Salas, D. Cabrera, J. Camarero, F. J. Teran and D. Serantes, J. Phys. Chem. C, 2015, 119, 15698–15706 CAS.
- I. Astefanoaei, I. Dumitru, H. Chiriac and A. Stancu, J. Appl. Phys., 2014, 115, 17B531 CrossRef.
- R. M. Patil, N. D. Thorat, P. B. Shete, S. V. Otari, B. M. Tiwale and S. H. Pawar, Mater. Sci. Eng. C, 2016, 59, 702–709 CrossRef CAS PubMed.
- H. Zhou, W. Qian, F. M. Uckun, L. Wang, Y. A. Wang, H. Chen, D. Kooby, Q. Yu, M. Lipowska, C. A. Staley, H. Mao and L. Yang, ACS Nano, 2015, 9, 7976–7991 CrossRef CAS PubMed.
- K. McNamara and S. A. M. Tofail, Phys. Chem. Chem. Phys., 2015, 17, 27981–27995 RSC.
- T. Tegafaw, W. Xu, M. W. Ahmad, J. S. Baeck, Y. Chang, J. E. Bae, K. S. Chae, T. J. Kim and G. H. Lee, Nanotechnology, 2015, 26, 365102 CrossRef PubMed.
- J. Chen, W.-J. Zhang, Z. Guo, H.-B. Wang, D.-D. Wang, J.-J. Zhou and Q.-W. Chen, ACS Appl. Mater. Interfaces, 2015, 7, 5373–5383 CAS.
- G. Zhang, R. Du, L. Zhang, D. Cai, X. Sun, Y. Zhou, J. Zhou, J. Qian, K. Zhong, K. Zheng, D. Kaigler, W. Liu, X. Zhang, D. Zou and Z. Wu, Adv. Funct. Mater., 2015, 25, 6101–6111 CrossRef CAS.
- R. Chen, D. Ling, L. Zhao, S. Wang, Y. Liu, R. Bai, S. Baik, Y. Zhao, C. Chen and T. Hyeon, ACS Nano, 2015, 9, 12425–12435 CrossRef CAS PubMed.
- L. Wang, X. Zhu, X. Tang, C. Wu, Z. Zhou, C. Sun, S.-L. Deng, H. Ai and J. Gao, Chem. Commun., 2015, 51, 4390–4393 RSC.
- L. Li, W. Jiang, K. Luo, H. Song, F. Lan, Y. Wu and Z. Gu, Theranostics, 2013, 3, 595–615 CrossRef PubMed.
- P. B. Shete, R. M. Patil, N. D. Thorat, A. Prasad, R. S. Ningthoujam, S. J. Ghosh and S. H. Pawar, Appl. Surf. Sci., 2014, 288, 149–157 CrossRef CAS.
- N. D. Thorat, R. A. Bohara, S. A. M. Tofail, Z. A. Alothman, M. J. A. Shiddiky, M. S. A Hossain, Y. Yamauchi and K. C.-W. Wu, Eur. J. Inorg. Chem., 2016 DOI:10.1002/ejic.201600706.
- T. N. Brusentsova, N. A. Brusentsov, V. D. Kuznetsov and V. N. Nikiforov, J. Magn. Magn. Mater., 2005, 293, 298–302 CrossRef CAS.
- S.-H. Liao, C.-H. Liu, B. P. Bastakoti, N. Suzuki, Y. Chang, Y. Yamauchi, F.-H. Lin and K. C.-W. Wu, Int. J. Nanomed., 2015, 10, 3315–3327 CrossRef CAS PubMed.
- A. I. Prasad, A. K. Parchur, R. R. Juluri, N. Jadhav, B. N. Pandey, R. S. Ningthoujam and R. K. Vatsa, Dalton Trans., 2013, 42, 4885 RSC.
- D. Fourmy, J. Carrey and V. Gigoux, Nanomedicine, 2015, 10, 893–896 CrossRef CAS PubMed.
- R. A. Bohara, N. D. Thorat, A. K. Chaurasia and S. H. Pawar, RSC Adv., 2015, 5, 47225–47234 RSC.
- N. D. Thorat, R. A. Bohara, V. Malgras, S. A. M. Tofail, T. Ahamad, S. M. Alshehri, K. C.-W. Wu and Y. Yamauchi, ACS Appl. Mater. Interfaces, 2016, 8, 14656–14664 Search PubMed.
- N. D. Thorat, O. M. Lemine, R. A. Bohara, K. Omri, L. El Mir and S. A. M. Tofail, Phys. Chem. Chem. Phys., 2016, 33, 941–951 Search PubMed.
- N. D. Thorat, S. V Otari, R. M. Patil, R. A. Bohara, H. M. Yadav, V. B. Koli, A. K. Chaurasia and R. S. Ningthoujam, Dalton Trans., 2014, 43, 17343–17351 RSC.
- M. Ashfaq, N. Verma and S. Khan, Mater. Sci. Eng. C, 2016, 59, 938–947 CrossRef CAS PubMed.
- M. Lu, C. Zhao, Q. Wang, G. You, Y. Wang, H. Deng, G. Chen, S. Xia, J. Zhao, B. Wang, X. Li, L. Shao, Y. Wu, L. Zhao and H. Zhou, Colloids Surf., B, 2016, 139, 171–179 CrossRef CAS PubMed.
- D. E. Gheorghe, L. Cui, C. Karmonik, A. Brazdeikis, J. M. Penaloza, J. K. Young, R. A. Drezek and M. Bikram, Nanoscale Res. Lett., 2011, 6, 554 CrossRef PubMed.
- B. P. Jacob, S. Thankachan, S. Xavier and E. M. Mohammed, Phys. Scr., 2011, 84, 45702 CrossRef.
- Y.-I. Kim, W. Bin Im, M. K. Jeon, Y.-H. Lee, K.-B. Kim and K.-S. Ryul, J. Nanosci. Nanotechnol., 2011, 11, 810–814 CrossRef CAS PubMed.
- N. Xiao, W. Gu, H. Wang, Y. Deng, X. Shi and L. Ye, J. Colloid Interface Sci., 2014, 417, 159–165 CrossRef CAS PubMed.
- K. Krieble, T. Schaeffer, J. A. Paulsen, A. P. Ring, C. C. H. Lo and J. E. Snyder, J. Appl. Phys., 2005, 97, 10F101 CrossRef.
- S. Dey, S. K. Dey, B. Ghosh, P. Dasgupta, A. Poddar, V. R. Reddy and S. Kumar, J. Appl. Phys., 2013, 114, 93901 CrossRef.
- Z. Li, M. Kawashita, N. Araki, M. Mitsumori, M. Hiraoka and M. Doi, J. Biomater. Appl., 2011, 25, 643–661 CrossRef CAS PubMed.
- N. D. Thorat, K. P. Shinde, S. H. Pawar, K. C. Barick, C. A. Betty and R. S. Ningthoujam, Dalton Trans., 2012, 41, 3060–3071 RSC.
- L. Gutiérrez, R. Costo, C. Grüttner, F. Westphal, N. Gehrke, D. Heinke, A. Fornara, Q. A. Pankhurst, C. Johansson, S. Veintemillas-Verdaguer and M. P. Morales, Dalton Trans., 2015, 44, 2943–2952 RSC.
- Y. Oh, N. Lee, H. W. Kang and J. Oh, Nanotechnology, 2016, 27, 115101 CrossRef PubMed.
- K. Vamvakidis, M. Katsikini, D. Sakellari, E. C. Paloura, O. Kalogirou and C. Dendrinou-Samara, Dalton Trans., 2014, 43, 12754–12765 RSC.
- T. Kondo, K. Mori, M. Hachisu, T. Yamazaki, D. Okamoto, M. Watanabe, K. Gonda, H. Tada, Y. Hamada, M. Takano, N. Ohuchi and Y. Ichiyanagi, J. Appl. Phys., 2015, 117, 17D157 CrossRef.
- E. Fantechi, C. Innocenti, M. Albino, E. Lottini and C. Sangregorio, J. Magn. Magn. Mater., 2015, 380, 365–371 CrossRef CAS.
- C. Munoz-Menendez, I. Conde-Leboran, D. Baldomir, O. Chubykalo-Fesenko and D. Serantes, Phys. Chem. Chem. Phys., 2015, 17, 27812–27820 RSC.
- V. M. Kalita, A. I. Tovstolytkin, S. M. Ryabchenko, O. V. Yelenich, S. O. Solopan and A. G. Belous, Phys. Chem. Chem. Phys., 2015, 17, 18087–18097 RSC.
- F. Wang, E. Peng, B. Zheng, S. F. Y. Li and J. M. Xue, J. Phys. Chem. C, 2015, 119, 23735–23742 CAS.
- R. M. Patil, P. B. Shete, N. D. Thorat, S. V. Otari, K. C. Barick, A. Prasad, R. S. Ningthoujam, B. M. Tiwale and S. H. Pawar, RSC Adv., 2014, 4, 4515–4522 RSC.
- M. Mahmoudi, S. Laurent, M. A. Shokrgozar and M. Hosseinkhani, ACS Nano, 2011, 5, 7263–7276 CrossRef CAS PubMed.
- Y. Cheng, M. Liu, R. Li, C. Wang, C. Bai and K. Wang, Biochim. Biophys. Acta, 1999, 1421, 249–260 CrossRef CAS.
- M. A. Dobrovolskaia, J. D. Clogston, B. W. Neun, J. B. Hall, A. K. Patri and S. E. McNeil, Nano Lett., 2008, 8, 2180–2187 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra20135k |
| This journal is © The Royal Society of Chemistry 2016 |