N,N′-Diaryl-perylene-3,9-diamine derivatives: synthesis, characterization and electroluminescence properties†
Abstract
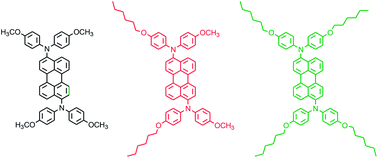
In this work, N-N′-diaryl-perylene-3,9-diamine (PDA) derivatives featuring different alkoxyl substituents at the para position of the N-aryl rings were synthesized and employed as emitters in solution processed organic light emitting diodes. Their physical properties were studied by UV-Vis absorption, fluorescence, differential scanning calorimetry, photo-electron yield spectroscopy and cyclic voltammetry, and were correlated with the OLED performance. Changing the length of the alkoxyl groups influences the PDAs' thermal properties, their bulk crystallinities and affects the performance of the respective OLEDs. The OLED emission spectra are red-shifted up to wavelengths of 800 nm, covering a spectral emission regime formerly inaccessible by other PDA derivatives.


 Please wait while we load your content...
Please wait while we load your content...