Synthesis of catalytically active gold clusters on the surface of Fe3O4@SiO2 nanoparticles†
Paulino Alonso-Cristobala,
M. Arturo Lopez-Quintela b,
Rafael Contreras-Caceresc,
Enrique Lopez-Cabarcosa,
Jorge Rubio-Retamaa and
Marco Laurenti
b,
Rafael Contreras-Caceresc,
Enrique Lopez-Cabarcosa,
Jorge Rubio-Retamaa and
Marco Laurenti *a
*a
aDepartment of Physical-Chemistry II, Faculty of Pharmacy, Universidad Complutense de Madrid, Madrid, Spain. E-mail: mlaurent@ucm.es; Tel: +34-913941751
bGrupo Nanomag, Instituto de Investigacións Tecnolóxicas, Universidade de Santiago de Compostela, Spain
cDepartment of Organic Chemistry, University of Malaga, Málaga, Spain
First published on 17th October 2016
Abstract
This work proposes a novel method to obtain catalytically active gold clusters by using the water-soluble 5,10,15,20-Tetrakis(4-trimethyl-ammonio-phenyl)porphyrin under mild conditions instead of using strong reducing agents. Uniform gold clusters were obtained with a diameter comprised between 1 and 2 nm and long-term stability. Furthermore, the water-soluble porphyrin was immobilized on the SiO2 shell of a core@shell Fe3O4@SiO2 nanoparticles to directly synthesize gold clusters onto the surface of Fe3O4@SiO2 nanoparticles featuring their magnetic recovery. Fe3O4@SiO2@Au nanoparticles were found to be catalytically active for the reduction of 4-nitrophenol to 4-aminophenol using NaBH4 and giving very high pseudo-first-order rate constant comprised between 0.7 and 2.7 min−1. Our results demonstrate that Fe3O4@SiO2@Au nanoparticles are stable catalysts and do not degrade during the catalytic process under the reaction conditions enabling their magnetic recuperation.
Introduction
Gold has been used for centuries in jewelry due to its stability against oxidation and considered not suitable as catalyst. Haruta et al. reported the first major discovery in the field of catalysis by gold nanoparticles (Au NPs) showing that nanosized gold catalyzed the oxidation of carbon monoxide to carbon dioxide at room temperature by atmospheric air.1 Nowadays, Au NPs have been recognized as an excellent catalyst and used in several processes to catalyze chemical reactions such as oxidation of alcohols2 and aldehydes,3 epoxidation of propylene,4 selective reduction of chloronitrobenzenes,5 and carbon–carbon bond formation.6 The catalytic property of gold is affected by several factors such as nanoparticle size, morphology, and metal-oxide support interaction.7–11 Unlike gold NPs whose optical properties are dominated by surface plasmon resonances, gold clusters possess molecule-like properties due to the quantum confinement effects resulting from “molecule-like” discrete orbital characteristics like HOMO–LUMO transition.12,13 For long time the Schmid's method remained the best way to synthesize gold clusters,14 however, in 1994 Brust and Schiffrin published a novel method to obtain gold clusters. This method had a considerable impact on the synthesis of gold clusters due to their stability, controlled size, and narrow dispersity.15 Today, Au clusters can be obtained by mass-selected gas-phase synthesis that permits a perfect control over the number of atoms.16 However, this method produces a very small amount of clusters that limits their catalytic applications. The reactivity of gold clusters is strongly affected by their atomicity and location on a solid support that would enhance Au clusters recovery making crucial their design.17–19 Current methods for the preparation of supported Au clusters require the immobilization of a precursor to a support and the removal of the ligands by post-synthesis treatment, trying to avoid cluster agglomeration during this step.20–22 Therefore, it is desirable to develop a methodology that permits to obtain large quantities of stable clusters directly immobilized on a solid support for their use and subsequently recovery. The deposition and encapsulation of Au clusters onto different substrates has been reported by several groups and it is used to enhance their colloidal stability.17,23 Haruta et al. reported the direct deposition of Au NPs on commercially available poly(methyl methacrylate) beads and their use for the catalytic reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) showing very interesting results.24 Chang et al. synthesized a magnetically recoverable Au catalyst by adsorption–reduction of Au3+ ions on chitosan-coated iron oxide nanoparticles. The catalytic activity of the magnetically recoverable Au catalyst was illustrated performing the reduction of 4-NP to 4-AP.25Porphyrins are molecules rich in π–π electrons and there is an extensive knowledge on the formation of complexes between porphyrins and Au3+ ions with different properties and applications.26–29 Sakamoto et al. proposed a novel strategy to obtain nano-platonic solids through the face coordination of porphyrin molecules on an inscribed Au nanoparticle with the adequate size.30 Recently, porphyrin molecules assembled onto a clay surface have been used for the deposition of gold clusters.31 However, up to day there is no report of direct synthesis of Au nanoparticles/clusters using water-soluble porphyrins.
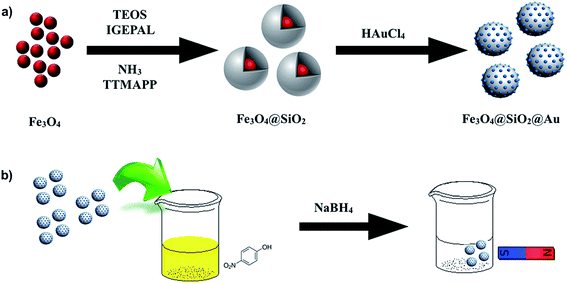
In this work, we report a simple strategy for the synthesis and immobilization of Au clusters onto the surface of a Fe3O4@SiO2 nanoparticles. Interestingly, we found that the water-soluble 5,10,15,20-Tetrakis(4-trimethyl-ammonio-phenyl)porphyrin used in this study is able to reduce HAuCl4 in a controlled manner resulting in the production of Au clusters. The encapsulation of the water-soluble porphyrin inside the silica shell of Fe3O4@SiO2 nanoparticles permitted the direct synthesis of Au clusters on the silica surface. The nanoparticles Fe3O4@SiO2@Au were used to catalyze the reduction of 4-NP to 4-AP with NaBH4 and the catalyst showed a high rate of conversion and the ability to maintain a 94% of catalytic activity for at least 6 cycles.
Materials and methods
Materials
5,10,15,20-Tetrakis(4-trimethyl-ammonio-phenyl)porphyrin tetra(p-toluenesulfonate) (TTMAPP) dye content 90%, 1-octadecene 90%, iron(III) citrate 99%, HAuCl4·3H2O ACS reagent ≥49.0% Au basis, Igepal® CO-520 average Mn 441, oleic acid technical grade 90%, hexane ReagentPlus® ≥99%, NaBH4 ReagentPlus® ≥99%, 4-nitrophenol reagent grade 98%, tetraethyl orthosilicate 98% (TEOS), methanol ACS reagent ≥99.5%, and acetone ACS reagent ≥99.5% were purchased from Sigma-Aldrich and used as received without any further treatment. NH3 29% was purchased from Panreac. TEM copper grid mesh-200 coated with formvar/carbon was purchased from 2SPI.Synthesis of hydrophobic Fe3O4 nanoparticles
In a 100 mL three-neck round bottom flask were added 10 mL of 1-octadecene, 1156 mg of oleic acid (4 mmol), and 245 mg of iron(III) citrate (1 mmol). The mixture was heated for one hour at 120 °C to remove any low boiling-point compounds of the solvent in an inert atmosphere of nitrogen. Then, the solution was heated for three hours at 305 °C to start the process of the thermal decomposition of the iron citrate. The color of the solution changed from brown to black indicating the formation of iron oxide nanoparticles. The reaction was cooled down at room temperature and the nanoparticles were precipitated by centrifugation at 8000 rpm with a solution of acetone/hexane 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, the supernatant was discarded and the solid redispersed. The process was repeated two times and the Fe3O4 nanoparticles were stored in hexane with a final concentration of 10 mg mL−1.
1 v/v, the supernatant was discarded and the solid redispersed. The process was repeated two times and the Fe3O4 nanoparticles were stored in hexane with a final concentration of 10 mg mL−1.
Synthesis of core@shell Fe3O4@SiO2 nanoparticles with encapsulated porphyrin
To a solution of 4.5 mL hexane were added 240 μL of Igepal® CO-520 (average Mn 441, 0.544 mmol) and 20 μL of hydrophobic Fe3O4 nanoparticles in hexane (10 mg mL−1). A solution of TTMAPP was prepared by dissolving 40 mg of porphyrin in 1 mL of NH3 29%. The ammonia solution (40 μL) was added to the Fe3O4 nanoparticles solution and vigorously mixed to obtain a homogenous microemulsion. Finally, 30 μL of TEOS was added under stirring and the reaction was continued for 18 hours. The addition of methanol disrupted the microemulsion and the porphyrin-encapsulated Fe3O4@SiO2 nanoparticles were centrifuged at 8000 rpm and the supernatant discarded. The core–shell nanoparticles were washed two additional times with ethanol, centrifuged, and finally dispersed in water at a concentration of 2 mg mL−1.Deposition of the Au clusters on the surface of the Fe3O4@SiO2 nanoparticles
25 μL of HAuCl4 30 mmol L−1 (0.75 mmol) were mixed with 5 mL of the porphyrin-encapsulated Fe3O4@SiO2 nanoparticles and kept at room temperature for 12 hours without stirring. After this time, the Au cluster decorated Fe3O4@SiO2 nanoparticles were centrifuged at 8000 rpm for 10 minutes, the supernatant was discarded to remove any unreacted HAuCl4 and any unanchored Au clusters/nanoparticles and the process was repeated two times. The Fe3O4@SiO2@Au solid was then redispersed in methanol at 2 mg mL−1.Transmission electron microscopy (TEM)
Au clusters and Fe3O4@SiO2 nanoparticles decorated with Au clusters were analyzed using high-resolution TEM JEM-2100 (JEOL Ltd. Tokyo, Japan) working at 200 kV with an ADF Gatan detector (Gatan Inc, Pleasanton, USA). TEM copper grids with mesh 200 covered with formvar/carbon and lacey formvar/carbon were purchased from 2SPI. TEM grid was prepared by deposition of a 10 μL drop of the different solutions and blotted after 90 seconds using filter paper. X-ray energy dispersive spectroscopy (EDS) analysis was performed using an Oxford INCA (Scanservice Corporation, Tustin, USA) device connected with the TEM microscope.X-ray photoelectron spectroscopy (XPS)
The chemical composition of the sample with Au clusters was examined by XPS surface measurements. In a typical experiment Au clusters were obtained as follow. 10 mL of a water solution of TTMAPP porphyrin (0.1 mmol L−1, 1 mmol) were placed in a 25 mL glass vial and mixed with 25 μL of HAuCl4 (30 mmol L−1, 1.5 mmol) and the color of the solution rapidly changed from red-wine to dark green. After 20 minutes from the beginning of the reaction, the color of the solution gradually changed to dark brown-orange typical of Au clusters. The solution was held without stirring for 3 hours at room temperature and finally centrifuged at 8000 rpm for 10 minutes in order to separate Au nanoparticles formed during the synthesis. The high-resolution XPS spectra C1s, O1s, Au4f, and survey spectra were recorded using a Thermo Scientific K-Alpha instrument (Thermo Fisher Scientific Inc, East Grinstead, UK). Monochromatic X-ray source Al Kα (1486.6 eV) was used for all samples and experiments. The X-ray monochromatic spot was 400 μm in diameter. Residual vacuum in the analysis chamber was maintained at around 6 × 10−10 mbar. The binding energies (BEs) positions were referenced to the C1s on unsputtered surfaces. Charge referencing was done by setting the binding energy of C1s hydrocarbon peak at 285.0 eV. High-resolution XPS spectra of Au4f were also acquired using the snapshot mode. When a spectrum is acquired in snapshot mode, the acquisition is faster allowing more acquisition with the same scan time and the spectra is high quality when the detector has a large number of channels, as in our case. We have also employed an electron flood gun to minimize surface charging. Avantage software (5.932v) was used to determine the atomic concentrations from the XPS peak areas using the Shirley background subtraction technique and the Scofield sensitivity factors.32,33 Some fragments of the samples were fixed to the sample holder with a metal clamp to ensure the electrical contact between holder and sample.Ultraviolet-visible (UV-vis) spectroscopy
UV-vis spectra of the free-base porphyrin before and after the reaction with HAuCl4 were taken using a Variant Cary-Bio300 at room temperature (Agilent, Santa Clara, Ca, USA). UV-vis was also used to monitor the catalytic reduction of 4-NP to 4-AP. The experiments were performed in a crystal quartz cuvette (path length 1 cm) mixing 200 μL of 4-NP (2 mmol L−1), 1.4 mL of NaBH4 (100 mmol L−1), different volumes of the dispersed Fe3O4@SiO2@Au (2 mg mL−1), and deionized water to reach a fixed volume of 3 mL.Results and discussions
The porphyrin structure is made of conjugated double bonds and they posses a typical UV-vis absorption spectrum displaying an extreme intense band in the range of 380–500 nm (Soret band) with a molar extinction coefficient of 105 mol L−1 cm−1, and the Q bands at longer wavelengths in the range of 500–750 nm with a lower extinction coefficient of 104 mol L−1 cm−1.34 Porphyrins are well known for their ability to form stable metalloporphyrin complexes and, depending on the ionic radius of the metal ion, the porphyrin complex can be in-plane (rion < 90 pm) or out-of-plane.34 The formation of metalloporphyrin complexes can be monitored using spectrophotometric techniques, such as UV-vis, following the displacement of the Soret band of the porphyrin. The formation of an in-plane porphyrin complex generates a blue shift of the Soret band respect to the free-base porphyrin in its UV-vis absorption spectra. The bathochromic shift is primarily due to the distortion of the macrocycle and not to the electronic properties of the metal.34 Au(III) has ionic radius of 85 pm and it can fit properly into the cavity of the porphyrin ring forming very stable in-plane complexes.27,34,35 For this reason, we monitored the evolution of the Soret and Q bands during the reaction between the TTMAPP porphyrin and HAuCl4. Fig. 1a shows the Soret band at 413 nm of the free-base TTMAPP porphyrin (red line) at neutral pH. After the addition of the first aliquot (5 μL) of HAuCl4 the intensity of the Soret band decreased conspicuously (black line) probably due to the redox reaction between the electron donor porphyrin and the acceptor HAuCl4. The addition of the second aliquot (5 μL) had the effect to slightly decrease the intensity of the Soret band (green line), however, it is also visible the presence of a shoulder on the left part of the band probably due to the formation and simultaneous presence of the Au(III)–TTMAPP complex. The third and forth addition of HAuCl4 (5 + 5 μL) provoked the complete blue-shift of the Soret band at 404 nm probably due to total transformation of the remaining free-base TTMAPP into Au(III)–TTMAPP complex, and the last addition of HAuCl4 (5 μL) decreased the intensity of the Soret band at 404 nm. The inset shows a magnification of the UV-vis spectra of the Q bands that confirms the contemporary presence of free-base TTMAPP and Au(III)–TTMAPP complex. Fig. 1b shows the reaction performed at acidic pH and the course of the reaction was different. The addition of 60 μL of HCl 10 mM caused the total red-shift of the Soret band from 413 to 432 nm (black line) due to the protonation of the pyrrole rings that induces a conformational change of the planar structure of the porphyrin ring.34 The first addition of 5 μL of HAuCl4 caused a small decreased of the Soret and Q bands, while further additions of HAuCl4 (20 μL) strongly decreased the intensity of the Soret and Q bands without the formation of Au(III)–TTMAPP complex. Fig. 1c and d show the TEM pictures of the gold clusters obtained with a diameter size of 1.01 ± 0.2 nm (Fig. S1†) and long-term stability using the two different conditions.The products obtained using the different acidic pH conditions and TTMAPP were further characterized using X-ray photoelectron spectroscopy (XPS) to determine the possible presence of different Au oxidation states (Au3+, Au+, and/or Au0). First, the solutions containing Au clusters were centrifuged to separate gold nanoparticles formed during the synthesis.
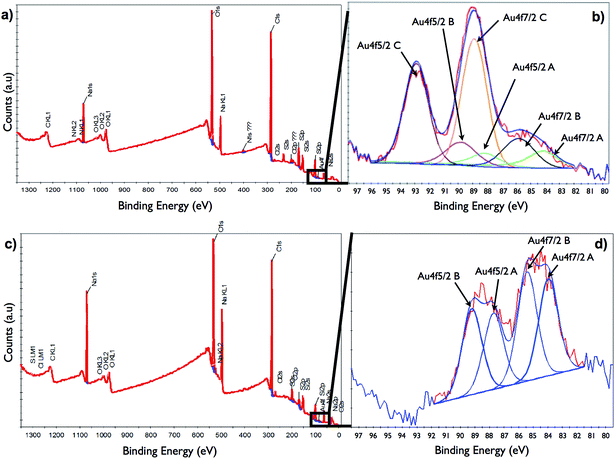
High-resolution XPS spectra of Au4f were acquired using the snapshot mode and used for the subsequent chemical state assignment of Au species. The XPS study of the sample prepared using the reaction between TTMAPP and HAuCl4 at neutral pH shows that Au was present in different oxidation states (Fig. 2a and b). The high-resolution spectrum of the Au4f core level was deconvoluted into three pairs of peaks due to Au4f7/2 and Au4f5/2 spin–orbit coupling. The first pair of peaks Au4f7/2A and Au4f5/2A at Binding Energies (BEs) of 83.6 and 87.6 eV is related to elemental gold (Au0), the other two pairs are related to the two gold oxide states Au+ (Au4f7/2B and Au4f5/2B at BEs of 85.3 and 89.3 eV) and Au3+ (Au4f7/2C and Au4f5/2C at BEs of 88.3 and 92.3 eV). On the basis of relative peak areas, their respective atomic percentages were estimated as 15% for Au0, 20% for Au+, and 65% for Au3+. For the reaction at acidic pH, the high-resolution XPS spectrum of the Au4f core level was deconvoluted obtaining two pairs of peaks due to Au4f7/2 and Au4f5/2 spin–orbit coupling (Fig. 2c and d). The first pair with BE at 83.9 and 88.0 eV are related to Au0 and the other pair is related to the stable gold oxide state, Au+ (BEs of 85.4 and 89.2 eV).36,37 On the basis of relative peak areas, their respective atomic percentages were estimated as 47% for Au0 and 53% for Au+ indicating higher conversion efficiency during the reaction between TTMAPP and HAuCl4 at acidic pH without the production of Au(III)–TTMAPP complex. To facilitate the purification of the Au clusters and provide a solid support that would allow their stability and recyclability, we synthesized Au clusters on the surface of Fe3O4@SiO2 nanoparticles using a synthetic approach shown in Scheme 1.
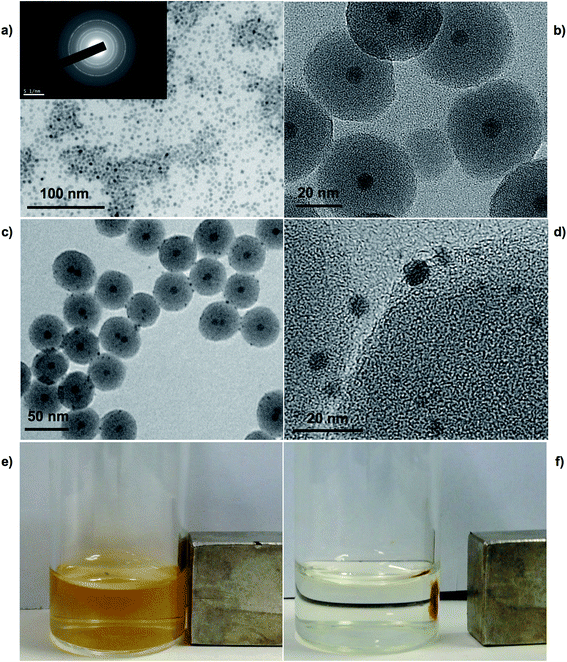
The first step required the synthesis of iron oxide nanoparticles obtained by thermal decomposition using as precursor iron(III) citrate.38 TEM image showed highly monodisperse iron oxide nanoparticles, which facilitates the next step of coating with a homogenous layer of SiO2, and with an average diameter of 10 ± 1 nm (Fig. 3a). SAED pattern analysis of the sample revealed the crystalline structure of the nanoparticles (Fig. 3a inset) and based on the measured lattice spacing based on the rings of the SAED pattern the result matched with the known lattice spacing of bulk Fe3O4 (ICDD card no. 19.0629) along with their respective hkl indexes (Table 1).
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| d (Å) | 2.98 | 2.51 | 2.07 | 1.61 | 1.48 |
| Fe3O4 | 2.97 | 2.53 | 2.1 | 1.62 | 1.48 |
| hkl | 220 | 311 | 400 | 511 | 440 |
The encapsulation of iron oxide nanoparticles within the silica was obtained through TEOS hydrolysis in a reverse microemulsion. The presence of negative charges on the surfaces of the silica shell provides colloidal stability to the Fe3O4@SiO2 nanoparticles.39,40 TTMAPP porphyrin was dissolved in NH3 29%, that is the aqueous phase of the reverse microemulsion, to make possible the encapsulation within the silica matrix, and to reduce the gold salt in the following step. The high aqueous solubility of the TTMAPP makes possible an efficient encapsulation of porphyrin molecules within the silica matrix.41 TEM image shows the core@shell structure of Fe3O4@SiO2 nanoparticles obtained in very high yield (Fig. S2†). The Fe3O4@SiO2 nanoparticles were monodisperse and the average diameter calculated using the TEM micrograph was 43 ± 4 nm. Finally, the Fe3O4@SiO2 nanoparticles were mixed with an aqueous solution of HAuCl4. TEM and HR-TEM showed the presence of small dots on the surface of the silica shell (Fig. 3c and d). However, the presence of Au clusters on the surface of the SiO2 nanoparticle does not limit the possibility that some of them growth inside the nano-channels existing on the SiO2 shell. The solution presents a light orange color indicating the presence of encapsulated TTMAPP and iron oxide nanoparticles that are responsive to an external magnetic field (Fig. 3e and f). HR-TEM image showed that the size of the Au clusters obtained was slightly bigger than the Au clusters synthesized in water ranging from 1.1 to 3.7 nm with a mean diameter of 1.45 ± 0.6 nm (Fig. S3†). EDS analysis was used to obtain the elemental composition of the system Fe3O4@SiO2@Au with an amount of Fe of 8.45%, Si of 89.29%, and Au of 2.26% confirming that the small dots on the surface of the silica shell were gold (Fig. S3†).
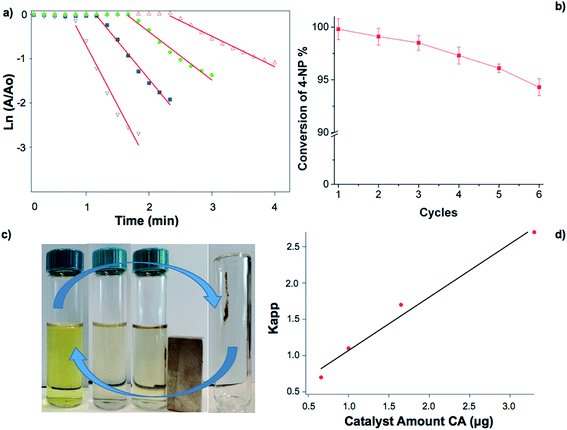
The catalytic reduction of 4-NP to the corresponding derivative 4-AP by NaBH4 was chosen as a model reaction to investigate the efficacy of Fe3O4@SiO2@Au.42–44 The reduction of 4-NP to 4-AP using aqueous NaBH4 is thermodynamically favorable (E0 for 4-NP/4-AP = −0.76 V and H3BO3/BH4− = −1.33 V versus NHE). However, the large potential difference between donor and acceptor decreases the feasibility of this reaction. Gold nanoparticles can catalyze this reaction by facilitating the electron transfer from the donor BH4− to the acceptor 4-NP and overcome the kinetic barrier of the reaction. The 4-NP shows an absorbance peak at 317 nm, which red shifts to 400 nm when is mixed with NaBH4 due to the formation of 4-nitrophenolate ions in the alkaline medium caused by NaBH4.45 Hence, the reaction progress can be followed by monitoring the decrease of the absorption at 400 nm of the 4-nitrophenolate ions by UV-vis spectroscopy. Since the concentration of NaBH4 (1.4 × 10−4 mol) greatly exceeds that of 4-NP (4.0 × 10−7 mol), the reduction rate can be assumed to be independent of NaBH4 concentration. Therefore, the apparent rate constant Kapp can be evaluated by studying the pseudo-first-order kinetics respect to the 4-NP concentration, and determined from the slope of the linear correlation of ln(A/A0) versus time (t), and is proportional to the total surface area S available.42,45 The reduction of 4-NP was conducted with different amounts of Fe3O4@SiO2@Au nanoparticles and the results are shown in Fig. 4a. Control experiments were performed to discard any catalytic effect of the TTMAPP porphyrin and/or Fe3O4@SiO2 nanoparticles and the results are shown in Fig. S4.† Liang et al. used iron oxide nanoparticles as catalyst for the reduction of 4-NP, however, the concentration of Fe3O4 nanoparticles necessary to observe a catalytic effect was much larger than gold as nanoparticles.46 From the control experiments, our system show no catalytic effect over the reduction of 4-NP during the observed time of 10 minutes. The linear relationship of ln(A/A0) versus time (t) indicates that the reduction of 4-NP by Fe3O4@SiO2@Au catalyst follows a pseudo-first-order kinetics. The calculated rate constants for 4-NP reduction were dependent on the amount of Fe3O4@SiO2@Au used to catalyze the reaction, as well as the induction time as reported in Table 2 and Fig. 4d that shows a dependence between the amount of catalyst and the calculated Kapp.
| Catalyst amount | Kapp (min−1) | Induction time (s) |
|---|---|---|
| 0.66 μg | 0.7 | 145 |
| 1.00 μg | 1.1 | 108 |
| 1.65 μg | 1.7 | 73 |
| 3.30 μg | 2.7 | 54 |
The rate constants for the reduction of 4-NP catalyzed by Fe3O4@SiO2@Au are higher than any previously reported values obtained by using Au nanoparticles or stabilized Au clusters.24,42,47–49 Kuroda et al. have used PMMA as a solid support for the deposition of gold nanoparticles and they reported a 4-NP reduction rate constant of 0.43 min−1 using 5.7 mg L−1 of catalyst.24 Gao et al. used a lower amount of catalyst 0.25 mg L−1 and the reduction rate constant was 0.44 min−1.47 Mahamallik et al. have used silica as solid support for the gold nanoparticles and they reported a 4-NP reduction rate of 0.25 min−1 using 50 mg L−1.48 Comparing the dose of catalyst used in our work, we used a minimum of 0.22 mg L−1 and a maximum of 1.1 mg L−1 of catalyst obtaining a 4-NP reduction rate constant ranging between 0.7 and 2.7 min−1. A different system such as the one proposed by Tzounis et al. showed that in the presence of SiO2@Ag nanoparticles they obtained a similar reduction rate constant, however, they used a much higher amount of catalyst.50 Comparing different nanoparticles having the same diameter, as the one reported by Xu et al., our system showed a superior performance. They used iridium oxide nanoparticles with a mean diameter of 1.7 nm to reduce 4-NP to 4-AP and the required time to achieve the total conversion was 25 minutes.51 Using 3.30 μg of catalyst the time required to achieve the total conversion of 4-NP to 4-AP was less than 1 minute. Our result seems to indicate that the Fe3O4@SiO2@Au nanoparticles are superior catalysts, enhancing the catalytic efficiency and minimizing the required amount of catalyst. It is noteworthy to point out that the induction time, t0, decreased when a higher amount of catalyst was used. The induction time has been reported by many groups during the catalytic reduction of 4-NP,42 however, its origin is not fully understood. Recently, Zhou et al. suggested that t0 is related to a dynamic restructuring of the surface of the nanoparticles.52 In fact, when a catalyst is introduced in the reaction media and it is not the active species, but this is formed during the reaction, an induction period should be observed.53 However, in heterogenous catalysis it is necessary to wait a certain time required for 4-NP to be adsorbed onto the surface of the catalyst and reach the equilibrium before the reaction begin. If this does not occur different induction times are required to reach the equilibrium using different amounts of Au catalyst. In our experiments a catalyst amount higher than 3.30 μg resulted in a t0 impossible to measure (data not shown). The reduction (and absence) of the induction time might indicate that the equilibrium was reached faster as a higher amount of catalyst was used, thus decreasing t0. The as-prepared Fe3O4@SiO2@Au nanoparticles showed excellent catalytic activity and were collected by an external magnet after the catalytic reduction for successive cycles. The use of Fe3O4@SiO2 as supporting material for the Au clusters permits the recovery, separation, and reuse of the catalyst. Fig. 4b shows the efficiency of conversion of the Fe3O4@SiO2@Au catalyst after six consecutive cycles and the conversion efficiency slightly decreased with the number of cycles. This result might be assessed to the loss of small amounts of Fe3O4@SiO2@Au nanoparticles during the magnetic separation and not to a significant change of the nanoparticles morphology and structure enabling their recyclability.
Conclusions
In summary, this is the first report of synthesis of water-stable Au clusters mediated by the redox reaction between the water-soluble TTMAPP porphyrins and HAuCl4 as shown by XPS, UV-vis and TEM. The synthesized Au clusters have a homogenous size distribution with a diameter of 1.01 ± 0.2 nm and very long-term stability. Furthermore, the Au clusters were directly synthesized onto a core@shell nanoparticle made of Fe3O4@SiO2 to produce a magnetically recoverable Au clusters. The iron oxide core provided magnetic responsiveness to the system, whereas the silica shell provided high colloidal stability to the system. The size of the Au clusters onto the Fe3O4@SiO2 nanoparticles was comprised between 1.1 and 3.7 nm with a mean diameter of 1.45 ± 0.6 nm. The Fe3O4@SiO2@Au nanoparticles were used as catalyst to study the reduction of 4-NP to 4-AP. The nanoparticles were able to catalyze the reaction giving a pseudo-first-order rate constant values comprised between 0.7 and 2.7 min−1. In addition the Fe3O4@SiO2@Au nanoparticles demonstrate to be a stable catalyst during the catalytic process enabling their recyclability due to the magnetic responsiveness of the Fe3O4@SiO2 nanoparticles.Competing financial interests
The authors declare no competing financial interests.Acknowledgements
The authors acknowledge the Spanish Ministry MINECO for financial support (MAT2014-55065R). M. A. L. Q. acknowledges the Spanish Ministry MINECO (MAT2012-36754-C02-01) and the Xunta de Galicia (GRC2013-044, FEDER Funds). P. A. C. acknowledges the Spanish Ministry of Education for the FPU fellowship (AP2010-1163).References
- M. Haruta, N. Yamada, T. Kobayashi and S. Iijima, Gold Catalysts Prepared by Coprecipitation for Low-Temperature Oxidation of Hydrogen and of Carbon-Monoxide, J. Catal., 1989, 115, 301–309 CrossRef CAS.
- R. L. Oliveira, P. K. Kiyohara and L. M. Rossi, High performance magnetic separation of gold nanoparticles for catalytic oxidation of alcohols, Green Chem., 2010, 12, 144–149 RSC.
- H. Y. Chen, C. T. Chang, S. J. Chiang, B. J. Liaw and Y. Z. Chen, Selective hydrogenation of crotonaldehyde in liquid-phase over Au/Mg2AlO hydrotalcite catalysts, Appl. Catal., A, 2010, 381, 209–215 CrossRef CAS.
- M. P. Kapoor, A. K. Sinha, S. Seelan, S. Inagaki, S. Tsubota, H. Yoshida and M. Haruta, Hydrophobicity induced vapor-phase oxidation of propene over gold supported on titanium incorporated hybrid mesoporous silsesquioxane, Chem. Commun., 2002, 2902–2903 RSC.
- Y. Y. Chen, J. S. Qiu, X. K. Wang and J. H. Xiu, Preparation and application of highly dispersed gold nanoparticles supported on silica for catalytic hydrogenation of aromatic nitro compounds, J. Catal., 2006, 242, 227–230 CrossRef CAS.
- J. Han, Y. Liu and R. Guo, Facile synthesis of highly stable gold nanoparticles and their unexpected excellent catalytic activity for Suzuki-Miyaura cross-coupling reaction in water, J. Am. Chem. Soc., 2009, 131, 2060–2061 CrossRef CAS PubMed.
- M. Haruta, Size- and support-dependency in the catalysis of gold, Catal. Today, 1997, 36, 153–166 CrossRef CAS.
- N. Lopez, T. V. W. Janssens, B. S. Clausen, Y. Xu, M. Mavrikakis, T. Bligaard and J. K. Norskov, On the origin of the catalytic activity of gold nanoparticles for low-temperature CO oxidation, J. Catal., 2004, 223, 232–235 CrossRef CAS.
- G. J. Hutchings, Catalysis by gold, Catal. Today, 2005, 100, 55–61 CrossRef CAS.
- T. Risse, S. Shaikhutdinov, N. Nilius, M. Sterrer and H. J. Freund, Gold supported on thin oxide films: from single atoms to nanoparticles, Acc. Chem. Res., 2008, 41, 949–956 CrossRef CAS PubMed.
- A. Corma, P. Concepcion, M. Boronat, M. J. Sabater, J. Navas, M. J. Yacaman, E. Larios, A. Posadas, M. A. Lopez-Quintela, D. Buceta, E. Mendoza, G. Guilera and A. Mayoral, Exceptional oxidation activity with size-controlled supported gold clusters of low atomicity, Nat. Chem., 2013, 5, 775–781 CrossRef CAS PubMed.
- S. Chen, R. S. Ingram, M. J. Hostetler, J. J. Pietron, R. W. Murray, T. G. Schaaff, J. T. Khoury, M. M. Alvarez and R. L. Whetten, Gold nanoelectrodes of varied size: transition to molecule-like charging, Science, 1998, 280, 2098–2101 CrossRef CAS PubMed.
- J. F. Parker, C. A. Fields-Zinna and R. W. Murray, The story of a monodisperse gold nanoparticle: Au25L18, Acc. Chem. Res., 2010, 43, 1289–1296 CrossRef CAS PubMed.
- G. Schmid, R. Pfeil, R. Boese, F. Bandermann, S. Meyer, G. H. M. Calis and W. A. Vandervelden, Au55[P(C6H5)3]12Cl6 – a Gold Cluster of an Exceptional Size, Chem. Ber/Recl., 1981, 114, 3634–3642 CrossRef CAS.
- M. Brust, M. Walker, D. Bethell, D. J. Schiffrin and R. Whyman, Synthesis of Thiol-Derivatized Gold Nanoparticles in a 2-Phase Liquid–Liquid System, J. Chem. Soc., Chem. Commun., 1994, 7, 801–802 RSC.
- H. Yasumatsu, T. Hayakawa, S. Koizumi and T. Kondow, Unisized two-dimensional platinum clusters on silicon(111)-7 × 7 surface observed with scanning tunneling microscope, J. Chem. Phys., 2005, 123, 124709 CrossRef PubMed.
- B. Santiago-Gonzalez, M. J. Rodriguez, C. Blanco, J. Rivas, M. A. Lopez-Quintela and J. M. Gaspar-Martinho, One Step Synthesis of the Smallest Photoluminescent and Paramagnetic PVP-Protected Gold Atomic Clusters, Nano Lett., 2010, 10, 4217–4221 CrossRef CAS PubMed.
- M. Stratakis and H. Garcia, Catalysis by Supported Gold Nanoparticles: Beyond Aerobic Oxidative Processes, Chem. Rev., 2012, 112, 4469–4506 CrossRef CAS PubMed.
- C. J. Jia and F. Schuth, Colloidal metal nanoparticles as a component of designed catalyst, Phys. Chem. Chem. Phys., 2011, 13, 2457–2487 RSC.
- M. L. Tran, A. V. Zvyagin and T. Plakhotnik, Synthesis and spectroscopic observation of dendrimer-encapsulated gold nanoclusters, Chem. Commun., 2006, 2400–2401 RSC.
- M. Turner, V. B. Golovko, O. P. H. Vaughan, P. Abdulkin, A. Berenguer-Murcia, M. S. Tikhov, B. F. G. Johnson and R. M. Lambert, Selective oxidation with dioxygen by gold nanoparticle catalysts derived from 55-atom clusters, Nature, 2008, 454, 981–983 CrossRef CAS PubMed.
- S. H. Xie, H. Tsunoyama, W. Kurashige, Y. Negishi and T. Tsukuda, Enhancement in Aerobic Alcohol Oxidation Catalysis of Au-25 Clusters by Single Pd Atom Doping, ACS Catal., 2012, 2, 1519–1523 CrossRef CAS.
- S. H. Joo, J. Y. Park, C. K. Tsung, Y. Yamada, P. D. Yang and G. A. Somorjai, Thermally stable Pt/mesoporous silica core–shell nanocatalysts for high-temperature reactions, Nat. Mater., 2009, 8, 126–131 CrossRef CAS PubMed.
- K. Kuroda, T. Ishida and M. Haruta, Reduction of 4-nitrophenol to 4-aminophenol over Au nanoparticles deposited on PMMA, J. Mol. Catal. A: Chem., 2009, 298, 7–11 CrossRef CAS.
- Y. C. Chang and D. H. Chen, Catalytic reduction of 4-nitrophenol by magnetically recoverable Au nanocatalyst, J. Hazard. Mater., 2009, 165, 664–669 CrossRef CAS PubMed.
- R. Jenkins, M. K. Burdette and S. H. Foulger, Mini-review: fluorescence imaging in cancer cells using dye-doped nanoparticles, RSC Adv., 2016, 6, 65459–65474 RSC.
- L. Z. He, T. F. Chen, Y. Y. You, H. Hu, W. J. Zheng, W. L. Kwong, T. T. Zou and C. M. Che, A Cancer-Targeted Nanosystem for Delivery of Gold(III) Complexes: Enhanced Selectivity and Apoptosis-Inducing Efficacy of a Gold(III) Porphyrin Complex, Angew. Chem., Int. Ed., 2014, 53, 12532–12536 CAS.
- I. Kim, H. M. Haverinen, Z. X. Wang, S. Madakuni, Y. Kim, J. Li and G. E. Jabbour, Efficient organic solar cell based on planar metallophthalocyanines, Chem. Mat., 2009, 21, 4256–4260 CrossRef CAS.
- T. Ema, Y. Miyazaki, J. Shimonishi, C. Maeda and J. Hasegawa, Bifunctional porphyrin catalysts for the synthesis of cyclic carbonates from epoxides and CO2: structural optimization and mechanistic study, J. Am. Chem. Soc., 2014, 136, 15270–15279 CrossRef CAS PubMed.
- M. Sakamoto, D. Tanaka, H. Tsunoyama, T. Tsukuda, Y. Minagawa, Y. Majima and T. Teranishi, Platonic Hexahedron Composed of Six Organic Faces with an Inscribed Au Cluster, J. Am. Chem. Soc., 2012, 134, 816–819 CrossRef CAS PubMed.
- T. Fujimura, Y. Yoshida, H. Inoue, T. Shimada and S. Takagi, Dense Deposition of Gold Nanoclusters utilizing Porphyrin/Inorganic Layered Material Complex as Template, Langmuir, 2015, 31, 9142–9147 CrossRef CAS PubMed.
- D. A. Shirley, High-Resolution X-Ray Photoemission Spectrum of the Valence Bands of Gold, Phys. Rev. B, 1972, 5, 4709 CrossRef.
- J. H. Scofield, Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV, J. Electron Spectrosc., 1976, 8, 129 CrossRef CAS.
- K. M. Kadish, K. Smith and R. Guilard, The Porphyrin Handbook, Academic Press, New York, 2000 Search PubMed.
- N. N. Greenwood and A. Earnshaw, Chemistry of the elements, Butterworth-Heinemann, Oxford, Boston, 1997 Search PubMed.
- Z. J. Wang, J. Zhang, Z. Y. Yin, S. X. Wu, D. Mandler and H. Zhang, Fabrication of nanoelectrode ensembles by electrodeposition of Au nanoparticles on single-layer graphene oxide sheets, Nanoscale, 2012, 4, 2728–2733 RSC.
- M. Boronat, P. Concepcion and A. Corma, Unravelling the nature of gold surface sites by combining IR spectroscopy and DFT calculations. Implications in catalysis, J. Phys. Chem. C, 2009, 113, 16772–16784 CAS.
- J. Park, K. J. An, Y. S. Hwang, J. G. Park, H. J. Noh, J. Y. Kim, J. H. Park, N. M. Hwang and T. Hyeon, Ultra-large-scale syntheses of monodisperse nanocrystals, Nat. Mater., 2004, 3, 891–895 CrossRef CAS PubMed.
- Y. Lu, Y. D. Yin, B. T. Mayers and Y. N. Xia, Modifying the surface properties of superparamagnetic iron oxide nanoparticles through a sol–gel approach, Nano Lett., 2002, 2, 183–186 CrossRef CAS.
- D. Serrano-Ruiz, P. Alonso-Cristobal, D. Mendez-Gonzalez, M. Laurenti, R. Olivero-David, E. Lopez-Cabarcos and J. Rubio-Retama, Nanosegregated Polymeric Domains on the Surface of Fe3O4@SiO2 Particles, J. Polym. Sci. Pol. Chem., 2014, 52, 2966–2975 CrossRef CAS.
- N. A. M. Verhaegh and A. Vanblaaderen, Dispersions of Rhodamine-Labeled Silica Spheres-Synthesis, Characterization, and Fluorescence Confocal Scanning Laser Microscopy, Langmuir, 1994, 10, 1427–1438 CrossRef CAS.
- P. Herves, M. Perez-Lorenzo, L. M. Liz-Marzan, J. Dzubiella, Y. Lu and M. Ballauff, Catalysis by metallic nanoparticles in aqueous solution: model reactions, Chem. Soc. Rev., 2012, 41, 5577–5587 RSC.
- J. Lin, Q. Chen, Y. N. Sun, M. Y. Xu, W. Liu and B. H. Han, Gold nanoparticles encapsulated in hierarchical porous polycarbazole: preparation and application in catalytic reduction, RSC Adv., 2016, 6, 48543–48549 RSC.
- M. Zhang, X. Lu, H. Y. Wang, X. Liu, Y. Qin, P. Zhang and Z. X. Guo, Porous gold nanoparticle/graphene oxide composite as efficient catalysts for reduction of 4-nitrophenol, RSC Adv., 2016, 6, 35945–35951 RSC.
- A. Gangula, R. Podila, M. Ramakrishna, L. Karanam, C. Janardhana and A. M. Rao, Catalytic Reduction of 4-Nitrophenol using Biogenic Gold and Silver Nanoparticles Derived from Breynia rhamnoides, Langmuir, 2011, 27, 15268–15274 CrossRef PubMed.
- J. Liang, A. Yue, Q. Wang, S. Song and L. Li, Tailored synthesis of well-faceted single crystals of Fe3O4 and their application in p-nitrophenol reduction, RSC Adv., 2016, 6, 29497–29503 RSC.
- Y. Gao, X. Ding, Z. Zheng, X. Cheng and Y. Peng, Template-free method to prepare polymer nanocapsules embedded with noble metal nanoparticles, Chem. Commun., 2007, 3720–3722 RSC.
- P. Mahamallik and A. Pal, A soft-template mediated approach for Au(0) formation on the heterosilica surface and synergism in the catalytic reduction of 4-nitrophenol, RSC Adv., 2015, 5, 78006–78016 RSC.
- A. Shivhare, S. J. Ambrose, H. X. Zhang, R. W. Purves and R. W. J. Scott, Stable and recyclable Au-25 clusters for the reduction of 4-nitrophenol, Chem. Commun., 2013, 49, 276–278 RSC.
- L. Tzounis, R. Contreras-Caceres, L. Schellkopf, D. Jehnichen, D. Fischer, C. Cai, P. Uhlmann and M. Stamm, Controlled growth of Ag nanoparticles onto the surface of SiO2 spheres: a nanohybrid system with combined SERS and catalytic properties, RSC Adv, 2014, 4, 17846–17855 RSC.
- D. Xu, P. Diao, T. Jun, Q. Y. Wu, X. F. Liu, X. Guo, H. Y. Gong, F. Li, M. Xiang and R. H. Yu, Iridium Oxide Nanoparticles and Iridium/Iridium Oxide Nanocomposites: Photochemical Fabrication and Application in Catalytic Reduction of 4-Nitrophenol, ACS Appl. Mater. Interfaces, 2015, 7, 16738–16749 CAS.
- X. C. Zhou, W. L. Xu, G. K. Liu, D. Panda and P. Chen, Size-Dependent Catalytic Activity and Dynamics of Gold Nanoparticles at the Single-Molecule Level, J. Am. Chem. Soc., 2010, 132, 138–146 CrossRef CAS PubMed.
- J. Oliver-Meseguer, J. R. Cabrero-Antonino, I. Dominguez, A. Leyva-Perez and A. Corma, Small Gold Clusters Formed in Solution Give Reaction Turnover Numbers of 107 at Room Temperature, Science, 2012, 338, 1452–1455 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra20055a |
| This journal is © The Royal Society of Chemistry 2016 |