Mechanism study on the photocatalytic efficiency enhancement of MoS2 modified Zn–AgIn5S8 quantum dots
Guan Gong†
,
Yanhong Liu†,
Baodong Mao*,
Bo Wang,
Lili Tan,
Di Li,
Yu Liu and
Weidong Shi*
School of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang 212013, P. R. China. E-mail: maobd@ujs.edu.cn; swd1978@ujs.edu.cn
First published on 3rd October 2016
Abstract
In this work, colloidal quantum dots (QDs) of environmentally friendly multinary semiconductor Zn–AgIn5S8 are used for heterostructure photocatalysts with hydrothermally deposited MoS2 focusing on the mechanism study of charge transfer processes in the photocatalytic system. The influence of MoS2 is systematically investigated on the structure, optical properties and photocatalytic activity of the Zn–AgIn5S8/MoS2 nanocomposites, where the compromise of the catalytic activity and light shielding effect and increased stability is observed with increasing MoS2. The optimized Zn–AgIn5S8/MoS2-5% composite exhibits high photocatalytic activity for nearly complete degradation of Rhodamine B within 4 min under visible light irradiation. The composite photocatalysts also show good activity on degradation of other organic pollutants including a colorless antibiotic, tetracycline. Photocatalytic mechanism study proves that the main oxidizing species is the superoxide radical formed by the reaction of photogenerated electrons and oxygen molecules, for which MoS2 mainly acts as a cocatalyst that promotes the electron transfer from the Zn–AgIn5S8 QDs. Time-resolved photoluminescence spectroscopy further reveals the increased charge separation by MoS2 from the photoluminescence quenching and lifetime decrease of the QDs, especially that of the long-lived trap states. Moreover, more efficient charge transfer from the dyes is also observed with Zn–AgIn5S8/MoS2, which is distinctively different from that with the Zn–AgIn5S8 QDs, indicating stronger interaction between the dye and photocatalyst with the introduction of MoS2. Based on these results, a continuous charge flow mechanism is proposed, where the electron transfer can be promoted by MoS2 not only from the QDs but also from the dyes that are all converted to superoxide radicals and contributed to the photocatalytic activity increase.
1. Introduction
Photocatalysis, as a widely studied sunlight-driven technique, has been considered as one of the most promising solutions for environment cleaning and renewable solar fuel production.1–3 Generally speaking, an efficient photocatalyst requires sufficient sun light absorption, especially in the visible range, and effective separation and utilization of the photogenerated charge carriers. In the past decades, extensive efforts have been devoted to the development of efficient visible light photocatalysts.4–6 Owing to the direct bandgap, high light absorption coefficient and low toxicity, multinary I–III–VI chalcogenide nanocrystals (NCs) (I = Cu, Ag; III = Al, In, Ga; VI = S, Se) have been comprehensively studied in the photocatalytic field,.7–11 More importantly, the I–III–VI NCs hold a unique advantage for energy conversion applications that their composition can be varied in a large range while the lattice structure being maintained, which renders them excellent tunability of the band gap and band energy positions.12–14 Photocatalytic degradation of organic dyes has been demonstrated for CuInS2,15 AgInS2 (ref. 16) and AgGaS2 (ref. 17) nanostructures. Various alloyed and doped I–III–VI NCs have also been developed for photocatalysis by alloying within the I–III–VI materials18 or alloying with other materials, such as ZnS19 and CdS,20 showing superior photocatalytic activity compared with the original counterparts. For example, AgIn5S8/AgInS2,21 ZnS–AgInS2,22,23 AgInxGa1−xS2,17 and AgInZnxSx+2 (ref. 24) NC based photocatalysts have been developed with composition dependent photocatalytic activity indicating a promising future for photocatalytic applications by composition and structure design. However, these I–III–VI NCs still suffer from the common stability issue of sulfide materials and large effort on the photocatalyst structure design is needed for activity and stability improvement for practical applications.A main consideration in photocatalyst design is the prompt separation and migration of the photogenerated electrons and holes, on which loading co-catalysts plays an important role.15,25–27 Among these materials, MoS2 is a 2D layered material with typical graphene-like structure that has attracted great attention in photocatalysis and electrocatalysis areas due to its low cost, high abundance and unique photoelectric properties.28–31 MoS2 has been combined with lots of semiconductors for photocatalytic applications, such as TiO2, Bi2MoO6, BiVO4, CdS and ZnIn2S4 etc., where it provides effective surface active sites that can prompt the surface catalytic reaction, inhibit recombination of photo-generated electrons and holes, and prevent the occurrence of counter reaction between active species and product.20,32–35 Moreover, MoS2 has a unique advantage for compositing with sulfide semiconductors that the shared S2− could reduce the lattice mismatch and defect states at the interfaces. On the other hand, since the active sites are mainly the exposed edge sites of the 2D S–Mo–S planes, recently more and more work has been contributed to the size control of MoS2 nanostructures towards high quality heterostructure photocatalysts with abundant active edge sites.20 These research works suggest a promising future of sulfide semiconductors combined with MoS2 for photocatalytic application. However, there has not been any report on size controlled MoS2 combined with I–III–VI NCs and more profound investigation on the structure and interface property manipulation is still needed towards a clear understanding of the role of MoS2 in the photocatalytic processes due to the complexity of the band energy alignment, structure, and deposition methods.
In the present study, series of Zn–AgIn5S8/MoS2 nanocomposites were prepared by combining MoS2 with monodispersed Zn–AgIn5S8 quantum dots (QDs) towards a more profound understanding of the role of MoS2 in photocatalyst design. The effect of the MoS2 content on the structure, optical properties and photocatalytic activity of the Zn–AgIn5S8/MoS2 nanocomposites was systematically investigated towards better understanding of the crucial role of MoS2 in the photocatalysis system. It was found that the visible-light-driven photocatalytic activity of Zn–AgIn5S8/MoS2 nanocomposites were dramatically enhanced compared to pure Zn–AgIn5S8 QDs. The related photocatalytic mechanism study indicates that the superoxide radicals (˙O2−) are the main active species with minor contribution from the holes. Steady state and time-resolved photoluminescence (PL) spectroscopies were used together for the first time to investigate the role of MoS2 in the charge transfer properties of the Zn–AgIn5S8/MoS2 photocatalysts in terms of PL quenching and lifetime change.
2. Experimental
2.1 Chemicals
All chemicals are analytical grade and used without further purification. Silver nitrate (AgNO3), zinc acetate (Zn(OAc)2·2H2O), indium nitrate (In(NO3)3·4.5H2O), thioacetamide (TAA), sodium hydroxide (NaOH), thiourea (CH4N2S), hexaammonium heptamolybdate ((NH4)6Mo7O24·4H2O), ethylenediamine tetraacetic acid (EDTA) dipotassium salt (C10H14K2N2O8·2H2O), Rhodamine B (RhB), tetracycline (TC), methyl orange (MO), methyl red (MR) and absolute ethanol were purchased from Sinopharm Chemical Reagent Co. Ltd. L-Cysteine (HSCH2CH(NH2)CO2H), 1,4-benzoquinone (BQ), isopropanol (IPA), and 5,5-dimethyl-1-pyrroline N-oxide (DMPO) were purchased from Aladdin (China).2.2 Synthesis of Zn–AgIn5S8 QDs and Zn–AgIn5S8/MoS2 composites
Pure Zn–AgIn5S8 QDs with Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) In
In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn ratio of 2
Zn ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 were prepared by a one-pot hydrothermal method based on previous report with revisions.19 In a typical synthesis process, 0.34 mmol of AgNO3, 1.7 mmol of In(NO3)3·4.5H2O, and 0.85 mmol of Zn(OAc)2·2H2O were added into 5.5 mL of water and a clear solution was obtained after stirring. Then 2 mL of 2.5 M L-cysteine solution was added under vigorous stirring. The pH value of the mixture was adjusted to 8.5 using 1.0 M NaOH solution and then 3.25 mmol of thioacetamide was rapidly added. The solution was transferred to a 35 mL Teflon-lined stainless steel autoclave, sealed and heated at 110 °C for 4 h. After naturally cooling down to room temperature, the samples were collected by ethanol precipitation and centrifugation and were further washed with water/ethanol cycles for three times. The cleaned QDs were dispersed in water for further characterization and photocatalytic tests.
5 were prepared by a one-pot hydrothermal method based on previous report with revisions.19 In a typical synthesis process, 0.34 mmol of AgNO3, 1.7 mmol of In(NO3)3·4.5H2O, and 0.85 mmol of Zn(OAc)2·2H2O were added into 5.5 mL of water and a clear solution was obtained after stirring. Then 2 mL of 2.5 M L-cysteine solution was added under vigorous stirring. The pH value of the mixture was adjusted to 8.5 using 1.0 M NaOH solution and then 3.25 mmol of thioacetamide was rapidly added. The solution was transferred to a 35 mL Teflon-lined stainless steel autoclave, sealed and heated at 110 °C for 4 h. After naturally cooling down to room temperature, the samples were collected by ethanol precipitation and centrifugation and were further washed with water/ethanol cycles for three times. The cleaned QDs were dispersed in water for further characterization and photocatalytic tests.
For preparation of Zn–AgIn5S8/MoS2 composite materials, 300 mg of the purified Zn–AgIn5S8 QDs were added to 30 mL of deionized water containing appropriate amounts of (NH4)6Mo7O24·4H2O and thiourea, where the molar ratio of Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S was kept at 7
S was kept at 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30. Subsequently, the solution was transferred to a 50 mL Teflon-lined stainless steel autoclave and maintained at 200 °C for 8 h. The purification procedure of Zn–AgIn5S8/MoS2 materials was the same as that of pure Zn–AgIn5S8 QDs. For comparison, a series of Zn–AgIn5S8/MoS2 composites were prepared with different amount of MoS2 ranging from 1 wt%, 3 wt%, 5 wt%, 10 wt%, 15 wt% to 30 wt%.
30. Subsequently, the solution was transferred to a 50 mL Teflon-lined stainless steel autoclave and maintained at 200 °C for 8 h. The purification procedure of Zn–AgIn5S8/MoS2 materials was the same as that of pure Zn–AgIn5S8 QDs. For comparison, a series of Zn–AgIn5S8/MoS2 composites were prepared with different amount of MoS2 ranging from 1 wt%, 3 wt%, 5 wt%, 10 wt%, 15 wt% to 30 wt%.
2.3 Characterizations
Structure of the samples was tested by X-ray diffraction (XRD, D8 ADVANCE, Bruker, Germany), using Cu-Kα radiation source (λ = 1.54056 Å) at a scanning rate of 4.0° per min. The transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) were also used to characterize the samples using a Tecnai G2 F30 S-Twin (FEI) electron microscope with an accelerating voltage of 200 kV. Raman spectra were recorded with an America ThermoFisher (DXR) Laser Raman Spectrometer with a 532 nm laser. The photoluminescence (PL) spectra of the samples were measured on a PerkinElmer LS 55 fluorescence spectrophotometer with an excitation wavelength of 450 nm. Time-resolved photoluminescence spectra were collected on a QuantaMaster™ 40 spectrometer (Photon Technology International, Inc.) with excitation wavelength of 481 nm. The electron spin resonance (ESR) spectra were collected on a Bruker EPR A300-10/12 spectrometer.2.4 Photocatalytic activity evaluation and mechanism study
The photocatalytic activities of the Zn–AgIn5S8/MoS2 composite materials were first evaluated via the photocatalytic degradation of RhB in aqueous solution under visible light irradiation. A 250 W Xe lamp with a 420 nm cutoff filter was used to provide visible light irradiation. In each experiment, 10 mg of photocatalyst was added into a 100 mL of the RhB solution (10 mg L−1). Prior to irradiation, the suspensions were stirred in the dark for 60 min to reach the adsorption–desorption equilibrium. After light irradiation, 4 mL of the suspension was extracted at 2 min intervals and centrifuged to obtain a clear liquid that will be used for concentration measurement. In addition, to further explore its photocatalytic application, the Zn–AgIn5S8/MoS2 composite photocatalysts were also investigated for degradation of different kinds of pollutants, including tetracycline (TC), methyl orange (MO) and methyl red (MR) with all the operations the same as that of RhB. Finally, the concentrations of RhB, TC, MO and TR were monitored using a TU-1810 UV-vis spectrometer at corresponding maximum absorption wavelengths of 553, 357, 463 and 410 nm, respectively. The dyes used in this work is mainly chosen in consideration of their hazard and the energy level alignment with the photocatalyst. With the rapid economic development, the pollution of wastewater from dyeing industry is the most dominant and tetracycline is an emerging antibiotic pollutant that is potentially dangerous for producing drug-resistant super bacteria. And RhB is further studied as the model dye for photodegradation mechanism because of its light absorption range and suitable energy levels that are suitable to investigate the interaction with our QDs based photocatalysts.To probe the active species involved in the photocatalysis process, photocatalytic degradation of RhB has been studied with the introduction of different kinds of scavengers, where EDTA36 as the hole scavenger, p-benzoquinone (BQ)37 as the superoxide radical scavenger, and isopropyl alcohol (IPA)38 as the hydroxyl radical scavenger. All other conditions were kept the same to the above photocatalytic experiments without scavengers. In addition, to further clarify the photocatalytic reaction mechanism, ESR technique was employed to detect the generated radicals in the photocatalytic system under visible light irradiation using 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as the radical trapping agent, which can trap the ˙O2− or ˙OH radicals and form DMPO–˙O2− or DMPO–˙OH complexes. Briefly, 2.5 mg of the Zn–AgIn5S8/MoS2 sample was dispersed in methanol (for ˙O2−) and then 30 μL of DMPO was added with ultrasonic dispersion for 3 min. ESR spectra of the trapped DMPO–˙O2− radicals were recorded before and after light irradiation.
3. Results and discussion
3.1 Structure and property characterization
XRD was used to characterize the phase of the as-prepared samples. As shown in Fig. 1a, the pure Zn–AgIn5S8 QDs basically match the characteristic peaks of cubic AgIn5S8 (JCPDS no. 25-1329).19 The peaks located at 27.3°, 46.6° and 55.0° can be assigned to the (311), (440) and (533) crystal planes of AgIn5S8, respectively. Owning to the small size of the particles, all of the diffraction peaks are relatively broad and weak. The top XRD pattern of MoS2 synthesized with similar conditions can be indexed to hexagonal phase MoS2 (JCPDS no. 73-1508) with the peaks of 29.5° and 58.5° assigned to the (101) and (110) crystal planes of MoS2, respectively.28 For the Zn–AgIn5S8/MoS2 composites, the XRD patterns are similar to that of the initial QDs with no noticeable characteristic MoS2 peak, even with the mass ratio of MoS2 increases from 1 wt% to 30 wt%. This may be due to the small amount and the poor crystallinity of MoS2 in the nanocomposites. Moreover, with respect to the invisibility of the MoS2 diffraction peaks in the heterojunctions, the highly similar XRD patterns of the Zn–AgIn5S8/MoS2 composites indicate that the low content of MoS2 did not bring any influence on the crystal structure of the Zn–AgIn5S8 QDs.Raman spectroscopy, as a more sensitive technique, was employed to further investigate the structure evolution of the Zn–AgIn5S8/MoS2 nanocomposites in comparison with the Zn–AgIn5S8 QDs and pure MoS2 nanosheets. As shown in Fig. 1b, the strong broad peaks around 318 (for Zn–AgIn5S8 QDs), 327 (for Zn–AgIn5S8/MoS2-1%) and 343 cm−1 (for Zn–AgIn5S8/MoS2-5%) are due to the PL signal of the Zn–AgIn5S8 QDs excited by the 532 nm green laser of the Raman spectrometer. Different from pure QDs, a tiny peak at 372 cm−1 was observed with 1 wt% and 5 wt% MoS2 that can be assigned to the E2g mode of MoS2. With the increase of MoS2 content, the PL peak gradually disappeared and the two Raman peaks at approximately 372 cm−1 and 402 cm−1 increased dramatically, which are assigned to the E2g and A1g modes of MoS2, respectively. There are no pronounced peak shift of the E2g and A1g modes compared to those of the pure MoS2 nanosheets.31 It is worth to mention that until MoS2 content was higher than 5%, the E2g and A1g modes of MoS2 fully appeared in the composites. The decrease of the PL peak of the QDs and increase of the E2g and A1g modes of MoS2 suggest the strong interaction between the two components and the dynamic growing process with increase of MoS2 ratio, which was further verified in the optical property studies presented below.
The morphology change of the Zn–AgIn5S8/MoS2 composites with different MoS2 content was investigated by TEM to study the hybridization process between the Zn–AgIn5S8 QDs and MoS2. As shown in Fig. 2a, the quaternary Zn–AgIn5S8 QDs prepared using a low temperature hydrothermal method show sphere-shaped morphology with average diameter of 4.3 ± 0.8 nm, exhibiting relatively uniform size with a narrow size distribution. Fig. 2b and c show the TEM images of the samples with 5 wt% and 30 wt% of MoS2, respectively. It can be seen that the pure Zn–AgIn5S8 QDs cover the surface of the MoS2 slices. With the increasing amount of MoS2, the nanocomposites showed size increase and the MoS2 nanosheets became more and more condensed as indicated by the characteristic stacked sheet structure of 2D layered materials (Fig. 2c). At the same time, the MoS2 slices also gradually coagulate together (Fig. 2c) instead of individual quantum size particles with increasing MoS2 content. As illustrated in Fig. 2d and e, the MoS2 nanosheets and Zn–AgIn5S8 QDs were strongly linked to each other, where the 2D sheet-like morphology of MoS2 with length over 50 nm already started to form. The enlarged image of Zn–AgIn5S8/MoS2-5% demonstrated that the Zn–AgIn5S8 QDs were attached to the MoS2 slices and an intimate interfaces were well formed between Zn–AgIn5S8 and MoS2 owning to the shared sulfide ions at the interface, which would favor the charge carrier transfer. The HRTEM images in Fig. 2d and e clearly presented the lattice spacing of 0.63 nm, which could be assigned as the (002) plane of MoS2,28 while the fringes with a lattice spacing of 0.33 nm correspond to the (311) plane of the Zn–AgIn5S8 QDs.19
 | ||
| Fig. 2 TEM images of the Zn–AgIn5S8/MoS2 samples with different mass ratios of MoS2: (a) 0 wt% (Zn–AgIn5S8 QDs), (b) 5 wt%, (c) 30 wt% and (d and e) HRTEM images of AgIn5S8/MoS2-5%. | ||
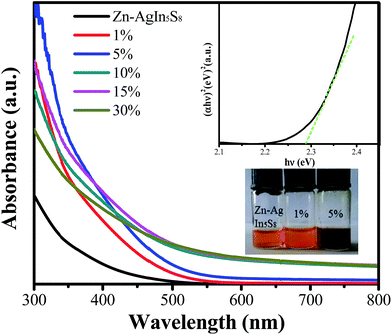
The optical property of Zn–AgIn5S8/MoS2 nanocomposites with increasing MoS2 ratio from 0 wt% to 30 wt% was examined using UV-vis absorption spectroscopy. As shown in Fig. 3, the absorption edge of the pure Zn–AgIn5S8 QDs was estimated to be ∼550 nm, while the Zn–AgIn5S8/MoS2 composite samples showed absorption edges of 600–650 nm, which may be ascribed to the increased visible light absorption caused by MoS2. Compared with pure Zn–AgIn5S8 QDs, the visible light absorption was improved dramatically with the increase of MoS2 content with colors changing from bright orange to dark red (lower inset of Fig. 3). The sample color change is also partly brought by increased light scattering with particle growth, changing from the clear orange solution (pure QDs) to the turbid orange state (1% MoS2) and further to dark red (5% MoS2). When the MoS2 content is higher than 5 wt%, further increase of light absorption was observed in the whole spectral range, which can be ascribed to the increased light scattering of the samples partly due to the size increase of composite particles. The UV-vis spectra show that the introduction of MoS2 enhanced the light absorption dramatically, though it may not necessarily contribute to the improvement of photocatalytic activity. In addition, the band gap (Eg) of pure Zn–AgIn5S8 QDs composites was estimated to be 2.28 eV from the plot of (αhν)2 vs. hν based on the UV/vis absorption data, as shown in the upper inset in Fig. 3.
Based on the above results, we propose a possible growing mechanism for the Zn–AgIn5S8/MoS2 nanocomposites (Scheme 1), where the amount of MoS2 has a major influence on both of the morphology and the optical properties. With lower MoS2 ratio (1 wt% and 5 wt%), the small MoS2 particles deposit on the surface of the QDs and the resulted Zn–AgIn5S8/MoS2 composite particles remain good colloidal stability along with a color change from orange to dark red (Fig. 3). Further increase of MoS2 results in the size increase of the composite particles due to the growth of the MoS2 2D slices and also brings high light absorption in a wide range and dark color. The influence of these results was further investigated in photocatalytic applications.
 | ||
| Scheme 1 Schematic illustration of the in situ growth process of MoS2 on the Zn–AgIn5S8 QDs for the construction of heterostructure photocatalysts. | ||
3.2 Photocatalytic activity
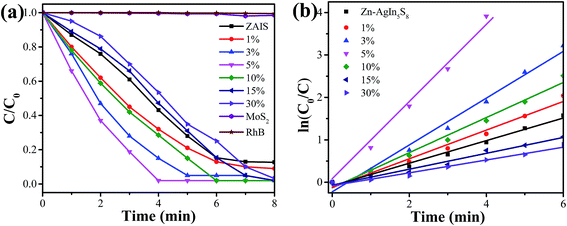
RhB was used as the probing molecule to explore the photocatalytic degradation activity of the Zn–AgIn5S8/MoS2 nanocomposites under visible-light irradiation. Fig. 4a shows the photocatalytic activities of the Zn–AgIn5S8/MoS2 nanocomposites with different MoS2 contents as well as the pure Zn–AgIn5S8 QDs and pure MoS2 nanosheets under visible-light irradiation. It can be found that RhB is only slightly degraded in the presence of MoS2 only or in the absence of various kinds of the prepared samples, indicating that the photocatalytic activity by MoS2 itself can be ignored and there is no noticeable decolorization of the dye without photocatalysts. Pure Zn–AgIn5S8 QDs showed relatively high photocatalytic activity for degradation of RhB (88%) under visible light within 8 min, which may be due to the small size that provides high surface area for the photocatalytic reaction to occur. Along with the introduction of different contents of MoS2, the photocatalytic performance of Zn–AgIn5S8/MoS2 heterojunction could be enhanced. The Zn–AgIn5S8/MoS2-5% composite shows the highest activity that RhB can be completely photodegraded in 4 min, which is much better than that of pure Zn–AgIn5S8 QDs. This degradation efficiency is much higher than that of previously reported AgIn5S8 powders (89.4% in 4 h for MO),39 monodispersed AgIn5S8 microspheres (98% in 20 min for MO) or ZnIn2S4/AgIn5S8 heteromicrospheres (99% in 50 min for RhB).40 The boosted photocatalytic activity may be attributed to the high surface area of the small size QD-based nanocomposites and the intimate interface between Zn–AgIn5S8 and MoS2 due to the shared S2− anions that can facilitate the interfacial charge transfer.20,32Moreover, the photocatalytic degradation kinetics results indicate that the reaction rate fit well with the pseudo-first order correlation as shown in Fig. 4b. It can be seen that the Zn–AgIn5S8/MoS2-5% sample exhibits the highest degradation rate, which is about 3.35 times higher than that of pure Zn–AgIn5S8 QDs. With increasing MoS2 content, the photocatalytic activity of the Zn–AgIn5S8/MoS2 composite materials was enhanced at first and then weakened. When the content of MoS2 is 1 wt% and 3 wt%, the photocatalytic activity has gradually enhanced comparing with the pure Zn–AgIn5S8 QDs. However, when MoS2 loading exceeds 5 wt%, the photocatalytic activity of the 10 wt%, 15 wt% and 30 wt% samples started to decrease gradually compared to the 5 wt% sample. The poorer photocatalytic activity of the composites than pure QDs is only observed with high ratio of MoS2 (15 wt% and 30 wt%). These results indicate that the optimal loading amount of Zn–AgIn5S8/MoS2 sample was 5 wt% in terms of photocatalytic activity, which suggests that MoS2 acts as a cocatalyst instead of light absorber and works best at relatively low loading amount. There are two contrast impacts with the introduction of MoS2: the increased charge separation (positive effect) and the increased light shielding (negative effect). The decrease of photocatalytic activity at high loading amount (10–30 wt%) may be due the strong light absorption of MoS2 that shielded the light absorption of the QDs similar to previously reported work.41 This observation indicates that the loading amount of MoS2 has to be compromised considering the catalytic activity enhancement and the light shielding effect.
In order to evaluate the stability and reusability of the Zn–AgIn5S8/MoS2 composite photocatalysts, cycle runs of photocatalytic degradation of RhB were carried out under visible-light irradiation. As shown in Fig. 5a, the efficiency of Zn–AgIn5S8/MoS2-5% decreases from 100% in the 1st run to 65% in the 3rd run, which may be due to the incomplete recycle of the small sized photocatalyst. On the other hand, the photocatalytic activity of Zn–AgIn5S8/MoS2-30% decreases from 100% in the 1st run to 90% in the 3rd run (Fig. 5b), e.g. the stability of the Zn–AgIn5S8/MoS2 photocatalysts was enhanced with the increase of MoS2. A plausible explanation is that the presence of extra MoS2 contributed to the efficient separation of the photo-generated electrons and holes. The photocatalytic activity of the AgIn5S8/MoS2-30% sample has a little deactivation (<10%) after three successive cycles for the degradation of RhB under visible-light irradiation, indicating that our photocatalyst possesses relatively high stability for practical applications.32
 | ||
| Fig. 5 Cycle runs of photocatalytic degradation of RhB under visible light irradiation over the Zn–AgIn5S8/MoS2 samples with 5 wt% (a) and 30 wt% (b) of MoS2. | ||
To further investigate their photocatalytic applications, the Zn–AgIn5S8/MoS2-5% sample was also evaluated for degradation of other dyes (MO and MR) and an antibiotic (TC). Fig. 6 shows the photocatalytic performance on the degradation of TC, MO and MR under visible light. It was found that the Zn–AgIn5S8/MoS2-5% nanocomposite could also efficiently decompose these organic pollutants, where TC could be degraded (74%) in 4 min and MO and MR could be completely degraded within 12 and 8 min. Hence, it is implied that our Zn–AgIn5S8/MoS2 photocatalysts display excellent photocatalytic activity in the visible-light range and has great potential in environment purification.
 | ||
| Fig. 6 Photocatalytic activity of the Zn–AgIn5S8/MoS2-5% nanocomposite for the degradation of TC, MO and MR under visible light irradiation. | ||
3.3 Photocatalytic mechanism study
In order to further reveal the reactive oxidation species formed over the Zn–AgIn5S8/MoS2 materials, ESR spectroscopy was performed after light irradiation. It is known that DMPO is generally used as a radical trapping agent forming DMPO–˙O2− or DMPO–˙OH.37,38 As shown in Fig. 7b, the twelve characteristic peaks of DMPO–˙O2− can be clearly observed in the methanol dispersion of Zn–AgIn5S8/MoS2-5% nanocomposite after visible light irradiation, indicating that the ˙O2− radicals were produced from the reaction of photogenerated electrons and O2 molecules in the photocatalytic systems. Combined with the trapping experiment (Fig. 7a), it can be inferred that ˙O2− played a major role in the photocatalytic system.
| ECB = X − Ee − 0.5Eg | (1) |
| EVB = ECB + Eg | (2) |
On the other hand, it was reported that the strong interaction between the dyes and photocatalysts plays an important role in lots of photocatalytic processes.4 In this Zn–AgIn5S8/MoS2 system, we propose that the dye/photocatalyst interaction can be effectively enhanced with the existence of MoS2 since the photogenerated electrons in the QDs can be promptly transferred. In the photocatalytic reaction, electrons from RhB-LUMO (−1.42 V vs. NHE)4 can be injected into the Zn–AgIn5S8 QDs conduction band (−0.74 V vs. NHE). The matching band energy levels (RhB, Zn–AgIn5S8 and MoS2) are shown in Scheme 2 and this synergistic effect are favorable for photogenerated electron–hole separation and transfer at both interfaces. To better demonstrate the effect of MoS2 in the photocatalytic system, the charge separation processes were further investigated by PL quenching and lifetime measurements for both of the QDs and RhB as discussed below.
To further probe the mechanism of electron–hole recombination in the Zn–AgIn5S8/MoS2 samples, time-resolved PL spectroscopy was used to assess the PL lifetimes (Fig. 8b). The PL decay curve of the prepared sample can be well fitted with the biexponential function:
I(t) = A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2 exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) exp(−t/τ2)
| (3) |
| τave = (A1τ1 + A2τ2)/(A1 + A2) | (4) |
| A1/% | τ1/ns | A2/% | τ2/ns | τave/ns | |
|---|---|---|---|---|---|
| Zn–AgIn5S8 QDs | 36.1 | 9.95 | 63.9 | 238.75 | 156.05 |
| Zn–AgIn5S8/MoS2-1% | 38.5 | 27.09 | 61.5 | 216.45 | 143.6 |
| Zn–AgIn5S8/MoS2-5% | 90.2 | 6.08 | 9.8 | 95.54 | 14.85 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 mixed solution (the condition of the photocatalytic reaction), but the quenching effect was slowed down with further addition of the QDs. In sharp contrast, nearly 100 times PL quenching of RhB by addition of Zn–AgIn5S8/MoS2-5% was observed, from 730 to below 10 for RhB
10 mixed solution (the condition of the photocatalytic reaction), but the quenching effect was slowed down with further addition of the QDs. In sharp contrast, nearly 100 times PL quenching of RhB by addition of Zn–AgIn5S8/MoS2-5% was observed, from 730 to below 10 for RhB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn–AgIn5S8/MoS2 = 1
Zn–AgIn5S8/MoS2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (Fig. 9b), e.g. nearly complete PL quenching in the actual photocatalytic reaction condition. Fig. 9c shows the relationship of the PL intensity vs. ratio of RhB/photocatalysts, which clearly indicates the much more efficient PL quenching by the Zn–AgIn5S8/MoS2 composite than the QDs. We propose that this enhanced interaction between RhB and Zn–AgIn5S8/MoS2 photocatalysts can be attributed to the MoS2 assisted electron transfer processes illustrated in Scheme 2.
10 (Fig. 9b), e.g. nearly complete PL quenching in the actual photocatalytic reaction condition. Fig. 9c shows the relationship of the PL intensity vs. ratio of RhB/photocatalysts, which clearly indicates the much more efficient PL quenching by the Zn–AgIn5S8/MoS2 composite than the QDs. We propose that this enhanced interaction between RhB and Zn–AgIn5S8/MoS2 photocatalysts can be attributed to the MoS2 assisted electron transfer processes illustrated in Scheme 2.
Further exploration by the time-resolved PL spectra of RhB with and without MoS2 gives more profound understanding of the charge separation and recombination processes. As shown in Fig. 9d, for RhB/Zn–AgIn5S8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) mixture, the PL intensity at 586 nm was decreased, but the lifetime remains almost unchanged (2.32 ± 0.04 ns) compared to that of the original RhB solution (2.31 ± 0.04 ns), which indicates that this is an energy transfer process rather than a charge transfer process. However, the PL lifetime of RhB was decreased from 2.31 ns to 1.91 ns when Zn–AgIn5S8/MoS2-5% was added, indicating a change of the excited state decay kinetics and the effective charge transfer from RhB to the Zn–AgIn5S8/MoS2-5% photocatalyst.44,45
4) mixture, the PL intensity at 586 nm was decreased, but the lifetime remains almost unchanged (2.32 ± 0.04 ns) compared to that of the original RhB solution (2.31 ± 0.04 ns), which indicates that this is an energy transfer process rather than a charge transfer process. However, the PL lifetime of RhB was decreased from 2.31 ns to 1.91 ns when Zn–AgIn5S8/MoS2-5% was added, indicating a change of the excited state decay kinetics and the effective charge transfer from RhB to the Zn–AgIn5S8/MoS2-5% photocatalyst.44,45
4. Conclusions
In summary, series of Zn–AgIn5S8/MoS2 nanocomposite photocatalysts were prepared by a facile hydrothermal method. The amount of MoS2 played a key role on the structure and optical property of the Zn–AgIn5S8/MoS2 nanocomposites and the compromise of catalytic activity enhancement and light shielding effect was observed with continuous growth of MoS2. Photocatalytic mechanism study indicates that the main oxidation species is superoxide radicals produced from the photogenerated electrons and oxygen molecules. The high photocatalytic degradation activity of organic pollutants by these composite photocatalysts can be attributed to the small size and the intimate interfacial contact that promotes the electron–hole separation and transfer. Moreover, stead state and time-resolved PL spectroscopy studies suggest that MoS2 mainly acts as a cocatalyst that promotes the electron transfer from the Zn–AgIn5S8 QDs. By further PL quenching and lifetime measurements of RhB upon the addition of Zn–AgIn5S8/MoS2 composites, it was found that MoS2 has a major influence on the interaction between the dye molecules and photocatalysts, for which a continuous charge flow mechanism was proposed with the introduction of MoS2. These findings provide an interesting view and useful guidance for the use of MoS2 in photocatalyst design in the future.Acknowledgements
The authors would like to acknowledge the supports from the National Natural Science Foundation of China (21501072 and 21522603), the Chinese-German Cooperation Research Project (GZ1091), the Distinguished Professor Program of Jiangsu Province, the Natural Science Foundation of Jiangsu Province (BK20150489), the “Innovative and Entrepreneurial Doctor” Program of Jiangsu Province, the China Postdoctoral Science Foundation (2016M590419), the Jiangsu Province Postdoctoral Foundation (1501027A), and the Start Funding of Jiangsu University (15JDG011 and 15JDG027).References
- F. Fresno, R. Portela, S. Suarez and J. M. Coronado, J. Mater. Chem. A, 2014, 2, 2863–2884 CAS.
- T. Hisatomi, J. Kubota and K. Domen, Chem. Soc. Rev., 2014, 43, 7520–7535 RSC.
- J. R. Ran, J. Zhang, J. G. Yu, M. Jaroniec and S. Z. Qiao, Chem. Soc. Rev., 2014, 43, 7787–7812 RSC.
- O. F. Lopes, E. C. Paris and C. Ribeiro, Appl. Catal., B, 2014, 144, 800–808 CrossRef CAS.
- H. Tong, S. Ouyang, Y. Bi, N. Umezawa, M. Oshikiri and J. Ye, Adv. Mater., 2012, 24, 229–251 CrossRef CAS PubMed.
- N. Razgoniaeva, P. Moroz, S. Lambright and M. Zamkov, J. Phys. Chem. Lett., 2015, 6, 4352–4359 CrossRef CAS PubMed.
- T. A. Kandiel, D. H. Anjum and K. Takanabe, ChemSusChem, 2014, 7, 3112–3121 CrossRef CAS PubMed.
- S. L. Shen and Q. B. Wang, Chem. Mater., 2013, 25, 1166–1178 CrossRef CAS.
- M. B. Wilker, K. J. Schnitzenbaumer and G. Dukovic, Isr. J. Chem., 2012, 52, 1002–1015 CrossRef CAS PubMed.
- J. K. Olesiak and H. Weller, ACS Appl. Mater. Interfaces, 2013, 5, 12221–12237 Search PubMed.
- M. D. Regulacio and M. Y. Han, Acc. Chem. Res., 2016, 49, 511–519 CrossRef CAS PubMed.
- T. Torimoto, T. Kameyama and S. Kuwabata, J. Phys. Chem. Lett., 2014, 5, 336–347 CrossRef CAS PubMed.
- B. D. Mao, C. H. Chuang, F. Lu, L. X. Sang, J. J. Zhu and C. Burda, J. Phys. Chem. C, 2013, 117, 648–656 CAS.
- I. Tsuji, H. Kato, H. Kobayashi and A. Kudo, J. Am. Chem. Soc., 2004, 126, 13406–13413 CrossRef CAS PubMed.
- X. N. Liu, Y. H. Tang, S. L. Luo, Y. Wang, X. L. Zhang, Y. Chen and C. B. Liu, J. Photochem. Photobiol., A, 2013, 262, 22–27 CrossRef CAS.
- B. Liu, X. Li, Q. Zhao, J. Ke, M. Tade and S. Liu, Appl. Catal., B, 2016, 185, 1–10 CrossRef CAS.
- N. Liang, Q. He, S. Huang, M. Wang, W. Chen, M. Xu, Y. Yuan, J. Zai, N. Fang and X. Qian, CrystEngComm, 2014, 16, 10123–10130 RSC.
- H. Kaga, Y. Tsutsui, A. Nagane, A. Iwase and A. Kudo, J. Mater. Chem. A, 2015, 3, 21815–21823 CAS.
- J. L. Q. Song, T. T. Jiang, T. Y. Guo, L. Liu, H. J. Wang, T. Y. Xia, W. T. Zhang, X. C. Ye, M. Y. Yang, L. X. Zhu, R. X. Xia and X. L. Xu, Inorg. Chem., 2015, 54, 1627–1633 CrossRef CAS PubMed.
- J. Z. Chen, X. J. Wu, L. S. Yin, B. Li, X. Hong, Z. X. Fan, B. Chen, C. Xue and H. Zhang, Angew. Chem., Int. Ed., 2014, 53, 1–6 CrossRef.
- X. Q. Li, D. L. Wei, S. Z. Kang and J. Mu, Adv. Mater. Res., 2013, 734–737, 2314–2317 Search PubMed.
- T. Kameyama, T. Takahashi, T. Machida, Y. Kamiya, T. Yamamoto, S. Kuwabata and T. Torimoto, J. Phys. Chem. C, 2015, 119, 24740–24749 CAS.
- T. Takahashi, A. Kudo, S. Kuwabata, A. Ishikawa, H. Ishihara, Y. Tsuboi and T. Torimoto, J. Phys. Chem. C, 2013, 117, 2511–2520 CAS.
- Y. Peng, L. Shang, Y. Cao, Q. Wang, Y. Zhao, C. Zhou, T. Bian, L. Z. Wu, C. H. Tung and T. Zhang, Appl. Surf. Sci., 2015, 358, 485–490 CrossRef CAS.
- Y. J. Yuan, H. W. Lu, Z. T. Yu and Z. G. Zou, ChemSusChem, 2015, 8, 4113–4127 CrossRef CAS PubMed.
- S. Bai, L. M. Wang, X. Y. Chen, J. T. Du and Y. J. Xiong, Nano Res., 2015, 8, 175–183 CrossRef CAS.
- Y. J. Yuan, Z. T. Yu, Y. H. Li, H. W. Lu, X. Chen, W. G. Tu, Z. G. Ji and Z. G. Zou, Appl. Catal., B, 2016, 181, 16–23 CrossRef CAS.
- J. F. Xie, H. Zhang, S. Li, R. X. Wang, X. Sun, M. Zhou, J. F. Zhou, X. W. Lou and Y. Xie, Adv. Mater., 2013, 25, 5807–5813 CrossRef CAS PubMed.
- Y. D. Hou, A. B. Laursen, J. S. Zhang, G. G. Zhang, Y. S. Zhou, X. C. Wang, S. Dahl and I. Chorkendorff, Angew. Chem., Int. Ed., 2013, 52, 3621–3625 CrossRef CAS PubMed.
- D. Gopalakrishnan, D. Damien and M. M. Shaijumon, ACS Nano, 2014, 8, 5297–5303 CrossRef CAS PubMed.
- B. D. Mao, Y. B. Yuan, Y. C. Shao, B. Yang, Z. G. Xiao and J. S. Huang, Nanosci. Nanotechnol. Lett., 2014, 6, 685–691 CrossRef CAS.
- G. H. Tian, Y. J. Chen, Z. Y. Ren, C. G. Tian, K. Pan, W. Zhou, J. Q. Wang and H. G. Fu, Chem.–Asian J., 2014, 5, 1291–1297 CrossRef PubMed.
- Y. Chen, G. Tian, Y. Shi, Y. Xiao and H. Fu, Appl. Catal., B, 2015, 164, 40–47 CrossRef CAS.
- W. Zhao, Y. Liu, Z. Wei, S. Yang, H. He and C. Sun, Appl. Catal., B, 2016, 185, 242–252 CrossRef CAS.
- Y. J. Yuan, H. W. Lu, Z. G. Ji, J. S. Zhong, M. Y. Ding, D. Q. Chen, Y. H. Li, W. G. Tu, D. P. Cao, Z. T. Yu and Z. G. Zou, Chem. Eng. J., 2015, 275, 8–16 CrossRef CAS.
- B. F. Luo, D. B. Xu, D. Li, G. L. Wu, M. M. Wu, W. D. Shi and M. Chen, ACS Appl. Mater. Interfaces, 2015, 7, 17061–17069 CAS.
- X. F. Wang, S. F. Li, Y. Q. Ma, H. G. Yu and J. G. Yu, J. Phys. Chem. C, 2011, 115, 14648–14655 CAS.
- U. A. Joshi, J. R. Darwent, H. P. Yiu and M. J. Rosseinsky, J. Chem. Technol. Biotechnol., 2011, 86, 1018–1023 CrossRef CAS.
- W. J. Zhang, D. Z. Li, M. Sun, Y. Shao, Z. X. Chen, G. C. Xiao and X. Z. Fu, J. Solid State Chem., 2010, 183, 2466–2474 CrossRef CAS.
- J. L. Q. Song, T. T. Jiang, G. W. Ji, W. T. Zhang, X. Y. Cheng, W. Weng, L. X. Zhu and X. L. Xu, RSC Adv., 2015, 116, 95943–95952 RSC.
- G. P. Chen, N. Ding, F. Li, Y. Z. Fan, Y. H. Luo, D. M. Li and Q. B. Meng, Appl. Catal., B, 2014, 160–161, 614–620 CrossRef CAS.
- B. D. Mao, C. H. Chuang, C. McCleese, J. J. Zhu and C. Burda, J. Phys. Chem. C, 2014, 118, 13883–13889 CAS.
- Y. H. Liang, S. L. Lin, L. Liu, J. S. Hu and W. Q. Cui, Appl. Catal., B, 2015, 164, 192–203 CrossRef CAS.
- X. Xiang, L. Chou and X. Li, Phys. Chem. Chem. Phys., 2013, 15, 19545–19549 RSC.
- A. Boulesbaa, Z. Huang, D. Wu and T. Lian, J. Phys. Chem. C, 2010, 114, 962–969 CAS.
- A. Boulesbaa, A. Issac, D. Stockwell, Z. Huang, J. Huang, J. Guo and T. Lian, J. Am. Chem. Soc., 2007, 129, 15132–15133 CrossRef CAS PubMed.
- K. Li, B. Chai, T. Y. Peng, J. Mao and L. Zan, ACS Catal., 2013, 3, 170–177 CrossRef CAS.
- K. W. Cheng and S. C. Wang, Mater. Chem. Phys., 2009, 115, 14–20 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |