Electrospraying magnetic-fluorescent bifunctional Janus PLGA microspheres with dual rare earth ions fluorescent-labeling drugs
Ping Liabc,
Kun Liabc,
Xufeng Niuabc and
Yubo Fan*abcd
aKey Laboratory for Biomechanics and Mechanobiology, Ministry of Education, Beihang University, 100191 Beijing, China
bSchool of Biological Science and Medical Engineering, Beihang University, 100191 Beijing, China
cBeijing Key Laboratory for Optimal Design and Evaluation Technology of Implantable & Interventional Medical Devices, 100191 Beijing, China
dNational Research Center for Rehabilitation Technical Aids, 100176 Beijing, China. E-mail: yubofan@buaa.edu.cn; Fax: +86-10-82339428; Tel: +86-10-82339428
First published on 3rd October 2016
Abstract
Magnetic-fluorescent bifunctional microspheres as a drug delivery system (DDS) possess magnetic targeting and fluorescent tracing capabilities simultaneously. The fluorescent intensity of DDS is weakened by magnetic particles, however; the fluorescent tracing of a drug cannot be achieved completely due to the separation of drug and fluorescent materials. In this study, we prepared a benzimidazole (Bim) fluorescent-labeling drug with dual rare earth (RE) ions as EuLa3(Bim)12 to increase the accuracy of tracing and fluorescent intensity. The electrospraying method with dual parallel spinnerets was used to produce Janus polylactide-co-glycolide (PLGA) microspheres with Fe3O4 nanoparticles and the fluorescent-labeling drug EuLa3(Bim)12 in separate chambers. Analysis results revealed that [PLGA/EuLa3(Bim)12]//[PLGA/Fe3O4] Janus microspheres simultaneously possessed superior magnetic and fluorescent properties. Moreover, the [PLGA/EuLa3(Bim)12]//[PLGA/Fe3O4] Janus microspheres showed higher fluorescent intensity than that of PLGA/EuLa3(Bim)12/Fe3O4 composite microspheres.
1. Introduction
Functional polymeric microspheres have unique chemical structures and morphology, and have garnered continually increasing research attention in regards to their potential application as a drug delivery system (DDS).1,2 This type of DDS could be used as a versatile platform for the delivery of a number of compounds ranging from small molecules to large macromolecules.3 Many physical or chemical methods have been applied in fabricating DDS microspheres, such as double emulsion,4 coacervation,5 and spray-drying.6Electrospraying based on electrospinning has noteworthy potential in regards to fabricating functional polymeric microspheres to deliver drugs. Electrospraying uses an electric field to break up liquid containing the material of interest into a continuous stream of finely dispersed particles;7,8 it is a one-step process that does not need any template or surfactant and can be carried out at ambient temperature and pressure. The microspheres made by electrospraying present good monodispersity under suitable conditions.9,10 Moreover, the morphology and size of the particles produced by electrospraying can be adjusted by controlling the electrospraying parameters such as solution concentration, flow rate, voltage, and collection distance.11–14 Electrospraying is also a versatile technique that can be used for preparing multifunctional drug carriers.
Multifunctional DDS has attracted a great deal of interest as well, as various functionalities (especially fluorescent and magnetic performance15) can be achieved in a single microsphere. The most common magnetic material is comprised of Fe3O4 nanoparticles, which have been applied in magnetic resonance imaging contrast agent, targeted localization, retinal detachment repair, and tumor magnetic heat treatment.16–18 Common fluorescent materials include organic fluorescent materials, light-emitting quantum dots, and rare earths (REs).19 The emitting character of RE elements has shown considerable potential in fluorescence detection,20 new materials,21 and bioimaging.22 Ma et al.23 prepared Fe3O4@Y203:Eu3+ magnetic-optical bifunctional composite nanoparticles by homogeneous precipitation method and applied them to detect cell separation. Fe3O4@nSiO2@mSiO2@NaYF4:Yb3+,Er3+/Tm3+ composite particles have also been fabricated as a drug delivery carrier by two-step sol–gel process.24
Researchers have found that black Fe3O4 magnetic nanoparticles (MNPs) can reduce fluorescent intensity in the magnetic-optical DDS due to the absorption of fluorescence. To obtain higher fluorescent intensity, the magnetic material Fe3O4 MNPs should be separated effectively from the fluorescent materials. Janus particles have two unique disparate nano-/micro chambers and can impart drastically distinguished chemical or physical properties and directionality within a single material.25–27 Electrospraying combining a two-phase side-by-side spinneret tip has been successfully used to generate Janus particles with different shapes in submicrons.28–32 Lahann produced bicompartmental bio-hybrid microparticles via electrohydrodynamic co-jetting of poly(acrylamide-co-acrylic acid) (PAAm-co-AA) solution containing FITC-conjugated dextran and acetylene-modified PAAm-co-AA solution containing rhodamine-conjugated dextran; the particles showed well-defined, spherical morphologies and two distinct compartments.30
In a previous study conducted in our laboratory, we prepared bifunctional DDS by incorporating fluorescent-labeling drugs and Fe3O4 MNPs into chitosan microspheres via water-in-oil microemulsion.5 The separation of fluorescent materials and drugs cannot reflect the delivery behavior of drugs precisely, as drugs may not release and diffuse along with the fluorescent substrate closely; the accuracy of fluorescent tracing can be ensured if the drugs are linked with the fluorescent materials. We applied RE ions Tb4+ as fluorescent label and synthesized the fluorescent-labeling drugs Tb(Bim)2(Asp)2 in order to increase the accuracy of drug delivery detection. Fe3O4 MNPs and fluorescent-labeling drugs Tb(Bim)2(Asp)2 were distributed evenly in the chitosan microspheres.
In this study, we applied an electrospraying setup with a parallel double-needle sprayer to prepare magnetic-fluorescent bifunctional Janus polylactide-co-glycolide (PLGA) microspheres with dual RE ions fluorescent-labeling drugs. RE ions Eu3+ and La3+ as fluorescent material and benzimidazole (Bim) as a model drug were adopted to prepare dual RE complexes EuLa3(Bim)12 by coordination reaction to reflect the delivery behavior of the drug directly. Europium complexes exhibit favorable luminescent properties due to the antenna ligands and f–f electron transition of Eu3+ ions.33–35 The second RE ion, La3+, was induced to form a dual-core RE complex to increase the fluorescence intensity.36–38 Bim was selected for its biological activity, e.g., antiviral, antibacterial, and antiparasitic properties.39 Magnetic-fluorescent bifunctional [PLGA/EuLa3(Bim)12]//[PLGA/Fe3O4] Janus microspheres as the DDS with different ratios of EuLa3(Bim)12 or Fe3O4 MNPs were synthesized, then the fluorescent intensities, magnetic properties, and morphologies of Janus microspheres and PLGA/EuLa3(Bim)12/Fe3O4 composite microspheres were analyzed at length as discussed below.
2. Experimental procedure
2.1 Materials
Ln2O3 (Ln = La, Eu) (99.99 wt%) and Bim (99.99 wt%) were purchased from Sinopharm Chemical Reagent Co., Ltd., China. NaOH, FeCl3·6H2O (≥99.0%) and FeCl2·4H2O (≥98.0%) were purchased from Xilong Chemical Co., Ltd., China. Hydrochloric acid (HCl, 36–38%) and chloroform (CHCl3, ≥99.0%) were purchased from Beijing Chemical Works, China. Polylactide-co-glycolide (PLGA, Mw = 1 × 105, LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GA = 85
GA = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) was obtained from Jinan Daigang Biotechnology Co., Ltd., China. Absolute ethyl alcohol (≥99.7%) was purchased from Modern Oriental Technology Development Co., Ltd., China.
15) was obtained from Jinan Daigang Biotechnology Co., Ltd., China. Absolute ethyl alcohol (≥99.7%) was purchased from Modern Oriental Technology Development Co., Ltd., China.
2.2 Synthesis of RE fluorescent-labeling drugs Eu(Bim)3, EuLa(Bim)6, EuLa3(Bim)12 and EuLa5(Bim)18
Five grams of Eu2O3 and 5 g La2O3 were dissolved in excessive concentrated hydrochloric acid (HCl) respectively. The solutions were heated for 1 h under magnetic stirring until RE oxides were dissolved completely. After 2 h of heating, white precipitates of EuCl3 or LaCl3 were formed in the solutions. Deionized water was used to wash the precipitates to remove excess chloride ions. To prepare complexes of Eu3+ and La3+ with Bim, EuCl3, LaCl3 and Bim were dissolved in absolute ethyl alcohol respectively under magnetic stirring to form homogenous solutions. When synthesizing single RE fluorescent-labeling drug Eu(Bim)3, Bim ethanol solution was added dropwise to EuCl3 ethanol solution with the mole ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the mixed solution was stirred magnetically at 60 °C for 4 h. To prepare the complexes of dual RE fluorescent-labeling drug EuLa(Bim)6, EuLa3(Bim)12 and EuLa5(Bim)18, Bim ethanol solution were added to EuCl3 and LaCl3 ethanol solution according to molar ratios of 1
1 and the mixed solution was stirred magnetically at 60 °C for 4 h. To prepare the complexes of dual RE fluorescent-labeling drug EuLa(Bim)6, EuLa3(Bim)12 and EuLa5(Bim)18, Bim ethanol solution were added to EuCl3 and LaCl3 ethanol solution according to molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12, 1
12, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 (Eu3+/La3+/Bim) respectively and the mixed solutions are stirred magnetically at 60 °C for 4 h. The RE complexes Eu(Bim)3, EuLa(Bim)6, EuLa3(Bim)12 and EuLa5(Bim)18 were formed as white precipitates once pH was adjusted to 6.5–7. Excess impurities were removed by centrifugation followed by washing with deionized water three times. The resultant solid materials were dried in a vacuum oven at 60 °C for 24 h. The final yields of Bim complexes were about 20%.
18 (Eu3+/La3+/Bim) respectively and the mixed solutions are stirred magnetically at 60 °C for 4 h. The RE complexes Eu(Bim)3, EuLa(Bim)6, EuLa3(Bim)12 and EuLa5(Bim)18 were formed as white precipitates once pH was adjusted to 6.5–7. Excess impurities were removed by centrifugation followed by washing with deionized water three times. The resultant solid materials were dried in a vacuum oven at 60 °C for 24 h. The final yields of Bim complexes were about 20%.
2.3 Synthesis of HCl modified Fe3O4 MNPs
FeCl3·6H2O (0.018 mol) and FeCl2·4H2O (0.01 mol) were dissolved in 100 mL deionized water, then 1.5 mol L−1 NaOH solution was added to the mixed solution and mechanically stirred for 1 h at 60 °C to obtain black precipitate. The precipitate was separated by centrifugation, then 0.03 M HCl solution was added and the solution was dispersed evenly by ultrasound.40 Finally, the black precipitate was washed several times with deionized water and dried under vacuum to obtain HCl-modified Fe3O4 MNPs.2.4 Preparation of magnetic-fluorescent bifunctional [PLGA/EuLa3(Bim)12]//[PLGA/Fe3O4] Janus microspheres and PLGA/EuLa3(Bim)12/Fe3O4 composite microspheres by electrospraying
| Sample | Method | PLGA/g | CHCl3/mL | EuLa3(Bim)12/g | Fe3O4 MNPs/g | |
|---|---|---|---|---|---|---|
| S1 | Double needles | A | 0.1 | 2.5 | 0.25 | |
| B | 0.1 | 2.5 | 0.10 | |||
| S2 | Double needles | A | 0.1 | 2.5 | 0.15 | |
| B | 0.1 | 2.5 | 0.10 | |||
| S3 | Double needles | A | 0.1 | 2.5 | 0.05 | |
| B | 0.1 | 2.5 | 0.10 | |||
| S4 | Double needles | A | 0.1 | 2.5 | 0.10 | |
| B | 0.1 | 2.5 | 0.10 | |||
| S5 | Double needles | A | 0.1 | 2.5 | 0.10 | |
| B | 0.1 | 2.5 | 0.05 | |||
| S6 | Double needles | A | 0.1 | 2.5 | 0.10 | |
| B | 0.1 | 2.5 | 0.02 | |||
| S7 | Single needle | 0.2 | 5.0 | 0.10 | 0.10 | |
Dispersion A PLGA/EuLa3(Bim)12 and dispersion B PLGA/Fe3O4 were injected into the two syringes of the double-needle electrospraying setup respectively, and the parallel sprayer was placed vertically. An aluminum foil supported by a lift table was used as the collector placed about 30 cm away from the tip of the stainless steel needle to collect the Janus microspheres. A positive direct current voltage of 8.5 kV was applied between the sprayer and collector. The flow rates of dispersions A and B were maintained at 0.4 mL h−1 during electrospraying. The electrospraying process was carried out at room temperature. For comparison, composite microspheres were also fabricated with a traditional single-needle electrospraying setup under the same preparation parameters.
2.5 Characterization
The structures of Bim, Eu(Bim)3, and EuLa3(Bim)12 were investigated by FTIR spectrometry (FTIR-650, Tianjin Gang Dong Technology Development Co., Ltd.) and UV/VIS/NIR spectrophotometry (UV-3600, Shimadzu, Japan). The fluorescent properties of Eu(Bim)3, EuLa(Bim)6, EuLa3(Bim)12, EuLa5(Bim)18, Janus microspheres and composite microspheres were detected with a fluorescence spectrophotometer (FS5, Edinburgh, UK). X-ray diffraction (XRD) patterns of prepared Fe3O4 MNPs, Bim, Eu(Bim)3, EuLa3(Bim)12, Janus microspheres and composite microspheres were measured with an X-ray diffractometer (XRD, D/MAX-2500, Rigaku, Japan) at 40 kV and 200 mA. The energy dispersive spectrum (EDS) of EuLa3(Bim)12 was observed under a field emission scanning electron microscope (FESEM, S-4800, Hitachi, Japan). The morphologies of Janus microspheres, composite microspheres, and Fe3O4 MNPs were observed by scanning electron microscopy (Quanta™ 250 FEG SEM, FEI, USA) and transmission electron microscope (JEM 1200EX TEM, NEC Electronics Corporation, Japan). The magnetic properties of Fe3O4 MNPs, Janus microspheres containing different quantities of Fe3O4 MNPs and composite microspheres were examined with a vibrating sample magnetometer (7307 VSM, Lake Shore, USA).3. Results and discussion
3.1 Infrared analysis
The FTIR spectra of Bim, Eu(Bim)3, and EuLa3(Bim)12 are shown in Fig. 2. The spectrum shown in Fig. 2(a) indicated that C–N stretching vibration, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration of Bim, C
N stretching vibration of Bim, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of Bim, and C–H out of plane bending vibration of the benzene ring occurred at 1464 cm−1, 1585 cm−1, 1620 cm−1, and 741 cm−1 respectively. The peak at 3061 cm−1 was caused by C–H stretching vibration of the benzene ring. According to the FTIR spectra of Eu(Bim)3 and EuLa3(Bim)12 shown in Fig. 2(b), the C–N and C
C stretching vibration of Bim, and C–H out of plane bending vibration of the benzene ring occurred at 1464 cm−1, 1585 cm−1, 1620 cm−1, and 741 cm−1 respectively. The peak at 3061 cm−1 was caused by C–H stretching vibration of the benzene ring. According to the FTIR spectra of Eu(Bim)3 and EuLa3(Bim)12 shown in Fig. 2(b), the C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration peaks of Bim showed a red shift from 1464 cm−1/1585 cm−1 to 1410 cm−1/1501 cm−1 due to the reduction of C
N stretching vibration peaks of Bim showed a red shift from 1464 cm−1/1585 cm−1 to 1410 cm−1/1501 cm−1 due to the reduction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibration force constant as Eu3+ was coordinated to the nitrogen atom of Bim in the complex. In the low-frequency region, a new peak at 541 cm−1 was observed due to the stretching vibration of Eu–N and La–N.41 The absorption intensities at 741 cm−1 and 1501 cm−1 of EuLa3(Bim)12 were higher than those of Eu(Bim)3, possibly owing to the induction of the second RE La3+. A wide hydroxyl characteristic vibration absorption peak was also observed at 3430 cm−1 indicating that the complex of Eu3+ with Bim may have contained H2O molecules.
N vibration force constant as Eu3+ was coordinated to the nitrogen atom of Bim in the complex. In the low-frequency region, a new peak at 541 cm−1 was observed due to the stretching vibration of Eu–N and La–N.41 The absorption intensities at 741 cm−1 and 1501 cm−1 of EuLa3(Bim)12 were higher than those of Eu(Bim)3, possibly owing to the induction of the second RE La3+. A wide hydroxyl characteristic vibration absorption peak was also observed at 3430 cm−1 indicating that the complex of Eu3+ with Bim may have contained H2O molecules.
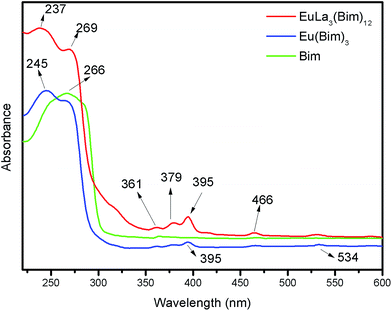
3.2 Ultraviolet analysis
The UV spectra of Bim, Eu(Bim)3, and EuLa3(Bim)12 are shown in Fig. 3. The peak located at 266 nm was due to the π–π* transition absorption of the aromatic ring in Bim. In the ultraviolet curves of coordination compound Eu(Bim)3 and EuLa3(Bim)12, absorption peaks at 237 nm and 245 nm stayed slightly blue-shifted compared to the absorption peak located at 266 nm in Bim.42 The peaks located at 380 nm and 395 nm of Eu(Bim)3 and EuLa3(Bim)12 were likely caused by d–d electron transition, i.e., ligand field transition of the central ion Eu3+,43 which suggests that Eu3+ was introduced in Eu(Bim)3 and EuLa3(Bim)12. The UV-absorption curve of Eu(Bim)3 was similar to that of EuLa3(Bim)12, suggesting that both products shared the same coordination mode.3.3 Fluorescent properties
The fluorescent properties of coordination compounds Eu(Bim)3, EuLa(Bim)6, EuLa3(Bim)12, and EuLa5(Bim)18 were assessed in addition to the fluorescent properties of magnetic-fluorescent bifunctional [PLGA/EuLa3(Bim)12]//[PLGA/Fe3O4] Janus microspheres containing various contents of EuLa3(Bim)12 or Fe3O4 MNPs and PLGA/EuLa3(Bim)12/Fe3O4 composite microspheres at room temperature.As shown in Fig. 4(a) and (c), the excitation spectra of Eu(Bim)3, EuLa(Bim)6, EuLa3(Bim)12 and EuLa5(Bim)18 contained obvious characteristic peaks at 395 nm when the emission wavelength was fixed at 620 nm corresponding to Eu3+ transition of 7F0–5L6. We accordingly used 395 nm as an excitation wavelength to obtain the emission spectra of Eu(Bim)3, EuLa(Bim)6, EuLa3(Bim)12 and EuLa5(Bim)18 as shown in Fig. 4(b) and (d).
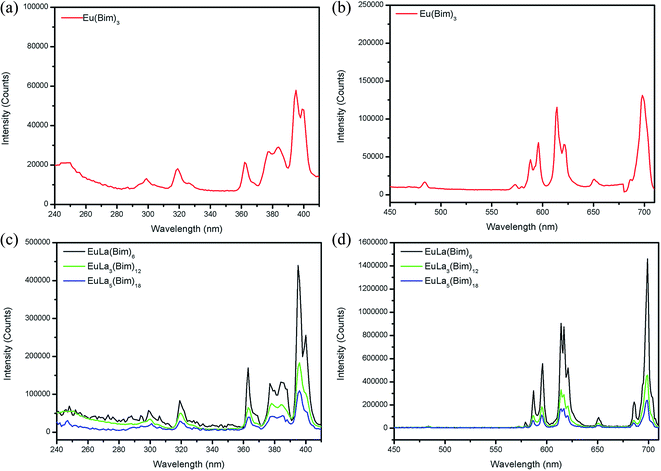 | ||
| Fig. 4 (a) Excitation spectrum and (b) emission spectrum of Eu(Bim)3; (c) excitation spectra and (d) emission spectra of EuLa(Bim)6, EuLa3(Bim)12 and EuLa5(Bim)18. | ||
The emission peaks of Eu(Bim)3, EuLa(Bim)6, EuLa3(Bim)12 and EuLa5(Bim)18 were located at 586 nm/595 nm (5D0–7F1), 614 nm/620 nm (5D0–7F2), and 686 nm/698 nm (5D0–7F4). The fluorescent intensities of EuLa(Bim)6, EuLa3(Bim)12 and EuLa5(Bim)18 were stronger than the fluorescence of Eu(Bim)3, as La3+ introduced in heteronuclears of EuLa(Bim)6, EuLa3(Bim)12 and EuLa5(Bim)18 provided a synergistic luminescence effect with Eu3+. The light absorbed by La3+ coordination was transmitted to Eu3+ coordination via intramolecular energy transfer; the total absorbed light of EuLa(Bim)6, EuLa3(Bim)12 and EuLa5(Bim)18 increased and thus the fluorescent intensity likewise increased.37,44
Fig. 4(d) presented that the fluorescent intensity of EuLa3(Bim)12 was higher than that of EuLa5(Bim)18, but lower than that of EuLa(Bim)6. When the content of La3+ was excess, the fluorescent intensity was reduced. The interaction between Eu3+ and La3+ was worth further studying. Although the fluorescent intensity of EuLa3(Bim)12 was lower than that of EuLa(Bim)6, EuLa3(Bim)12 contained higher content of drugs. In consideration of fluorescent intensity and the content of loaded drugs, we chose EuLa3(Bim)12 as the fluorescent-labeling drug in preparing Janus and composite microspheres.
As shown in Fig. 5, 395 nm was used as an excitation wavelength to obtain the emission spectra of Janus microspheres and composite microspheres. When the mass ratio of Fe3O4 MNPs to EuLa3(Bim)12 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the fluorescence intensity of Janus microspheres was much stronger than that of composite microspheres. In the composite microspheres, Fe3O4 MNPs and EuLa3(Bim)12 were distributed uniformly in the single chamber and black Fe3O4 MNPs absorbed the fluorescence of EuLa3(Bim)12. Conversely, in the Janus microspheres, Fe3O4 MNPs and EuLa3(Bim)12 were distributed in different chambers and thus immensely weakened the influence caused by Fe3O4 MNPs on fluorescence intensity.
1, the fluorescence intensity of Janus microspheres was much stronger than that of composite microspheres. In the composite microspheres, Fe3O4 MNPs and EuLa3(Bim)12 were distributed uniformly in the single chamber and black Fe3O4 MNPs absorbed the fluorescence of EuLa3(Bim)12. Conversely, in the Janus microspheres, Fe3O4 MNPs and EuLa3(Bim)12 were distributed in different chambers and thus immensely weakened the influence caused by Fe3O4 MNPs on fluorescence intensity.
As shown in Fig. 6, the emission spectra of Janus microspheres with various quantities of EuLa3(Bim)12 were obtained at excitation wavelength of 395 nm. The fluorescent intensities of Janus microspheres increased gradually as EuLa3(Bim)12 content rose from 0.05 g to 0.25 g while the mass of Fe3O4 MNPs was fixed at 0.1 g. The highest fluorescence intensity was acquired when the content of EuLa3(Bim)12 reached 0.25 g.
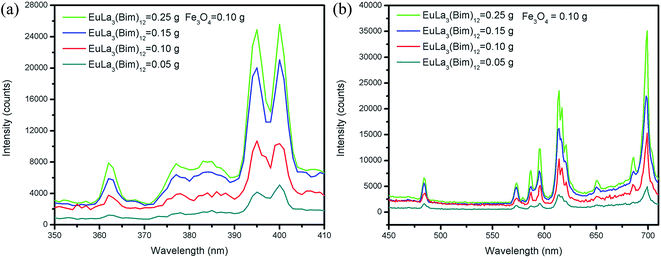 | ||
| Fig. 6 (a) Excitation spectra; (b) emission spectra of [PLGA/EuLa3(Bim)12]//[PLGA/Fe3O4] Janus microspheres with various EuLa3(Bim)12 at 0.1 g Fe3O4 MNPs mass. | ||
Fig. 7 depicted the fluorescence intensities of Janus microspheres as they increased gradually with decreasing Fe3O4 MNPs content (from 0.1 g to 0.02 g) when the mass of EuLa3(Bim)12 was fixed at 0.10 g and the excitation wavelength to 395 nm. The highest fluorescent intensity of Janus microspheres was obtained when Fe3O4 MNPs content was 0.02 g.
 | ||
| Fig. 7 (a) Excitation spectra; (b) emission spectra of [PLGA/EuLa3(Bim)12]//[PLGA/Fe3O4] Janus microspheres with different mass Fe3O4 MNPs at 0.1 g EuLa3(Bim)12. | ||
In summary, the magnetic-fluorescent bifunctional [PLGA/EuLa3(Bim)12]//[PLGA/Fe3O4] Janus microspheres exhibited higher fluorescent intensity than that of PLGA/EuLa3(Bim)12/Fe3O4 composite microspheres. The fluorescence intensity of Janus microspheres strengthened with increase in EuLa3(Bim)12 and decrease in Fe3O4 MNPs contents.
3.4 XRD and EDS tests
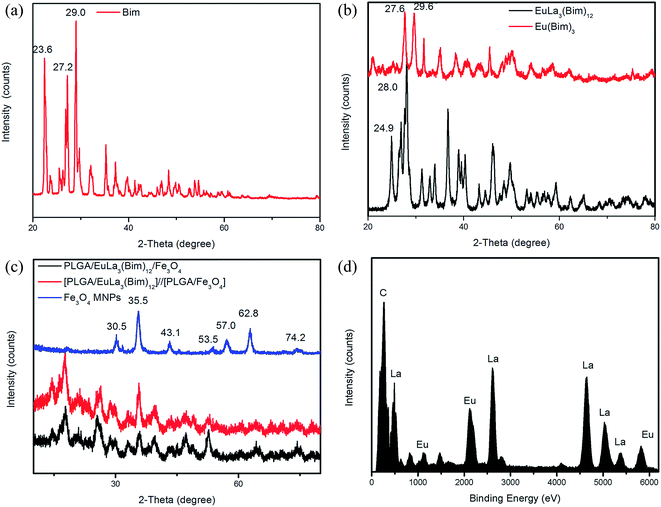
As shown in Fig. 8(a), Bim showed strong diffraction peaks located at 23.6°, 27.2°, 29.0°, 35.2°, 37.3°, and 48.4°, which confirms the favorable crystallization properties of Bim. The diffraction patterns of Eu(Bim)3 and EuLa3(Bim)12 are shown in Fig. 8(b). Compared to Bim, the peaks of Eu(Bim)3 showed slight shifts from 27.2°/29.0° to 27.6°/29.6° and the sharp peak at 23.6° disappeared. In comparison with Bim, the peaks of EuLa3(Bim)12 exhibited slight shifts from 23.6°/27.2° to 24.9°/28.0° and the sharp peak at 29.0° disappeared. The shifted peaks of Eu(Bim)3 and EuLa3(Bim)12 reflected changes in Bim crystallization properties and occurred likely due to the induction of RE ions Eu3+ and La3+. The intensities of diffraction peaks of EuLa3(Bim)12 were higher than those of Eu(Bim)3, possibly due to the increase in EuLa3(Bim)12 crystallinity caused by La3+ induction.As shown in Fig. 8(c), [PLGA/EuLa3(Bim)12]//[PLGA/Fe3O4] Janus microspheres showed similar diffraction peaks to PLGA/EuLa3(Bim)12/Fe3O4 composite microspheres. Peaks at 30.5°, 35.5°, 43.1°, 53.5°, 57.0°, 62.8°, and 74.2° corresponded respectively to the (220), (311), (400), (422), (511), (440), and (533) reflection planes of the face-centered cubic Fe3O4 crystal of Fe3O4 MNPs, which confirmed that Fe3O4 MNPs were wrapped into the microspheres successfully. Peaks at 24.9°, 28.0°, 46.2°, 49.7°, 59.3°, and 70.5° indicated that the EuLa3(Bim)12 were also wrapped into the microspheres.
The EDS spectrum of EuLa3(Bim)12 shown in Fig. 8(d) revealed that the Bim complex EuLa3(Bim)12 consisted of Eu, La, and C elements.
3.5 Magnetic properties
The magnetic performance of Fe3O4 MNPs, [PLGA/EuLa3(Bim)12]//[PLGA/Fe3O4] Janus microspheres and PLGA/EuLa3(Bim)12/Fe3O4 composite microspheres are shown in Fig. 9 and Table 2. Fig. 9(a) indicated that the Fe3O4 MNPs exhibited superparamagnetism with no magnetic remnants and zero coercion. Its saturation magnetization reached 65.53 emu g−1. As shown in Fig. 9(b), the saturation magnetization of Janus microspheres increased as Fe3O4 MNPs mass increased. As shown in Fig. 9(c), the saturation magnetization of Janus microspheres was similar to that of composite microspheres when the contents of Fe3O4 MNPs were both 0.1 g. The different structure of Janus microspheres and composite microspheres did not influence their respective magnetic properties, indicating good magnetic responsiveness. | ||
| Fig. 9 Hysteresis loops of (a) Fe3O4 MNPs, (b) [PLGA/EuLa3(Bim)12]//[PLGA/Fe3O4] Janus microspheres, and (c) PLGA/EuLa3(Bim)12/Fe3O4 composite microspheres. | ||
| Samples | Saturation magnetization (Ms) emu g−1 | |
|---|---|---|
| Fe3O4 MNPs | 65.53 | |
| Janus microspheres | Fe3O4 = 0.10 g | 5.7722 |
| Fe3O4 = 0.05 g | 2.1473 | |
| Fe3O4 = 0.02 g | 1.4848 | |
| Composite microspheres | Fe3O4 = 0.10 g | 5.6210 |
3.6 Morphologies
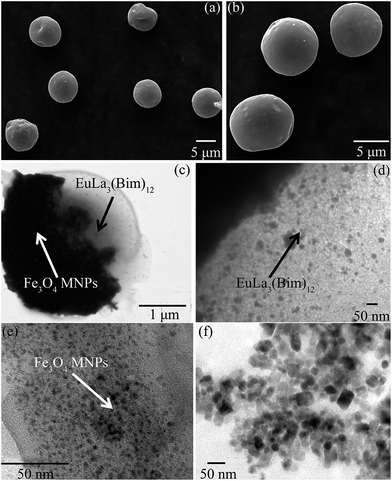
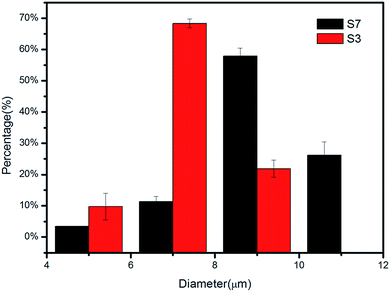
As shown in Fig. 10(a) and (b), the morphologies of [PLGA/EuLa3(Bim)12]//[PLGA/Fe3O4] Janus microspheres (S3) and PLGA/EuLa3(Bim)12/Fe3O4 composite microspheres (S7) showed regular spheres and with smooth microsphere surfaces. The diameter of S3 in Fig. 10(a) was less than that of S7 in Fig. 10(b). Particle diameter distribution histograms of samples S3 and S7 are shown in Fig. 11, where the diameter distribution of S3 is more uniform than the others. As shown in Fig. 10(c), Janus microspheres exhibited typical “Janus” structure with Fe3O4 MNPs and EuLa3(Bim)12 distributed in different chambers. Both Fe3O4 MNPs and EuLa3(Bim)12 showed excellent dispersity in PLGA microspheres under higher magnification as demonstrated in Fig. 10(d) and (e).TEM image of Fe3O4 MNPs is shown in Fig. 10(f), where the diameter of Fe3O4 MNPs is clearly less than 10 nm with favorable dispersity. Said dispersity was attributed to the modification of HCl. In the acidic solution, the surface of Fe3O4 MNPs absorbed a substantial amount of H+ causing the surface of ions to tend toward a positive charge and thus exhibit good dispersity due to electrostatic repulsion.40
4. Conclusion
In this study, we synthesized the DDS of magnetic-fluorescent, bifunctional [PLGA/EuLa3(Bim)12]//[PLGA/Fe3O4] Janus PLGA microspheres with dual RE ions as a fluorescent-labeling drug via electrospraying method. To enhance the fluorescent tracing accuracy, fluorescence-labeling drugs were synthesized by coordination reaction to ensure successful linking of the drug and fluorescent marker. Dual RE fluorescence-labeling drug EuLa3(Bim)12 showed higher fluorescent intensity than that of single RE fluorescence-labeling drug Eu(Bim)3. In magnetic-fluorescent Janus PLGA microspheres with EuLa3(Bim)12 as a fluorescent marker, fluorescent properties were satisfactory when 0.25 g EuLa3(Bim)12 and 0.02 g Fe3O4 MNPs were used. The Janus microspheres exhibited better fluorescent performance than PLGA/EuLa3(Bim)12/Fe3O4 composite microspheres. The Janus microspheres also showed favorable magnetic responsiveness, and EuLa3(Bim)12 and Fe3O4 particles were evenly distributed in separate chambers. This magnetic-fluorescent, bifunctional Janus PLGA microspheres with dual RE ions fluorescent-labeling drug has notable potential application in the DDS, biological imaging, and bioprobe fields.Acknowledgements
This work was supported by the National Science Foundation of China (11472032, 31470915, 61227902, 11120101001 and 11421202), National Basic Research Program of China (973 Program, 2011CB710901), 111 Project (B13003), Research Fund for the Doctoral Program of Higher Education of China (20131102130004), and International Joint Research Center of Aerospace Biotechnology and Medical Engineering, Ministry of Science and Technology, China.References
- C. Lin, L. Li, H. Zhang, W. Liu, Y. Yang, X. Liu and B. Xu, RSC Adv., 2014, 4, 46806–46812 RSC.
- X. Rong, Y. Xie, X. Hao, T. Chen, Y. Wang and Y. Liu, Curr. Drug Discovery Technol., 2011, 8, 173–187 CrossRef CAS.
- S. Freiberg and X. Zhu, Int. J. Pharm., 2004, 282, 1–18 CrossRef CAS PubMed.
- N. Bock, M. Woodruff, D. Hutmacher and T. Dargaville, Polymers, 2011, 3, 131–149 CrossRef CAS.
- P. Li, Y. Song, C. Liu, X. Li, G. Zhou and Y. Fan, Mater. Lett., 2014, 114, 132–135 CrossRef CAS.
- R. Pareta and M. Edirisinghe, J. R. Soc., Interface, 2006, 3, 573–582 CrossRef CAS PubMed.
- I. Hayati, A. Bailey and T. Tadros, Nature, 1986, 319, 41–43 CrossRef.
- A. Jaworek, Powder Technol., 2007, 176, 18–35 CrossRef CAS.
- X. Wang, X. Ju, S. Sun, R. Xie, W. Wang, Z. Liu and L. Chu, RSC Adv., 2015, 5, 34243–34250 RSC.
- C. Hu, J. Zhao and W. Cui, J. Appl. Polym. Sci., 2013, 128, 3177–3183 CrossRef CAS.
- P. Deotare and J. Kameoka, Nanotechnology, 2006, 17, 1380–1383 CrossRef CAS.
- S. Natha, S. Son, A. Sadiasa, Y. Min and B. Lee, Int. J. Pharm., 2013, 443, 87–94 CrossRef PubMed.
- E. Marjan, A. Zeeshan and S. Eleanor, J. R. Soc., Interface, 2010, 7, 667–675 CrossRef PubMed.
- A. Bohr, J. Kristensen, M. Dyas, M. Edirisinghe and E. Stride, J. R. Soc., Interface, 2012, 9, 2437–2449 CrossRef CAS PubMed.
- S. Labbaf, S. Deb, G. Gama, E. Stride and M. Edirisinghe, J. Colloid Interface Sci., 2013, 409, 245–254 CrossRef CAS PubMed.
- Y. Sun, L. Duan, Z. Guo, Y. Mu, M. Ma, L. Xu, Y. Zhang and N. Gu, J. Magn. Magn. Mater., 2005, 285, 65–70 CrossRef CAS.
- G. Li, Rare Met. Mater. Eng., 2005, 5, 673–675 Search PubMed.
- M. Aviles, H. Chen, A. Ebner, A. Rosengart, M. Kaminski and J. Ritter, J. Magn. Magn. Mater., 2007, 311, 306–311 CrossRef CAS.
- J. Kim, H. Kim, N. Lee, T. Kim, H. Kim, T. Yu, I. Song, W. Moon and T. Hyeon, Angew. Chem., Int. Ed., 2008, 47, 8438–8441 CrossRef CAS PubMed.
- J. Chen, F. Yi, H. Yu, S. Jiao, G. Pang and Z. Sun, Chem. Commun., 2014, 50, 10506–10509 RSC.
- G. Gou, R. Ren, S. Li, S. Guo, Z. Dong, M. Xie and J. Ma, New J. Chem., 2013, 37, 3861–3864 RSC.
- X. Wang, J. Chen, H. Zhu, X. Chen and X. Yan, Anal. Chem., 2013, 85, 10225–10231 CrossRef CAS PubMed.
- Z. Ma, D. Dosev, M. Nichkova, S. Gee, B. Hammock and I. Kennedy, J. Mater. Chem., 2009, 19, 4695–4700 RSC.
- S. Gai, P. Yang, C. Li, W. Wang, Y. Dai, N. Niu and J. Lin, Adv. Funct. Mater., 2010, 20, 1166–1172 CrossRef CAS.
- A. Walther and A. Müller, Chem. Rev., 2013, 113, 5194–5261 CrossRef CAS PubMed.
- L. Hong, S. Jiang and S. Granick, Langmuir, 2006, 22, 9495–9499 CrossRef CAS PubMed.
- T. Nisisako, T. Torii, T. Takahashi and Y. Takizawa, Adv. Mater., 2006, 18, 1152–1156 CrossRef CAS.
- K. Roh, D. Martin and J. Lahann, Nat. Mater., 2005, 4, 759–763 CrossRef CAS PubMed.
- S. Bhaskar, K. Roh, X. Jiang, G. Baker and J. Lahann, Macromol. Rapid Commun., 2008, 29, 1655–1660 CrossRef CAS.
- M. Yoshida, K. Roh, S. Mandal, S. Bhaskar, D. Lim, H. Nandivada, X. Deng and J. Lahann, Adv. Mater., 2009, 21, 4920–4925 CrossRef CAS PubMed.
- K. Roh, D. Martin and J. Lahann, J. Am. Chem. Soc., 2006, 128, 6796–6797 CrossRef CAS PubMed.
- K. Roh, M. Yoshida and J. Lahann, Langmuir, 2007, 23, 5683–5688 CrossRef CAS PubMed.
- S. Gai, P. Yang, D. Wang, C. Li, N. Niu, F. He, M. Zhang and J. Lin, RSC Adv., 2012, 2, 3281–3287 RSC.
- W. Dai, M. Zhou, Z. Xian and L. Zeng, RSC Adv., 2014, 4, 25470–25478 RSC.
- R. Gruar, C. Tighe, J. Muir, J. Kittler, M. Wodjak, A. Kenyon and J. Darr, RSC Adv., 2012, 2, 10037–10047 RSC.
- Y. Zhao and F. Zhao, Chin. J. Lumin., 2002, 3, 273–276 Search PubMed.
- Y. Zhao, F. Zhao, Z. Xue and L. Yan, Chin. J. Lumin., 2006, 3, 358–362 Search PubMed.
- D. Liu, A. Yan and A. Wu, Chin. J. Lumin., 1999, 3, 194–199 Search PubMed.
- H. Lv, X. Chen, L. Sun and S. Ye, J. Org. Chem., 2010, 75, 6973–6976 CrossRef CAS PubMed.
- Y. Ding, F. Liu, C. Cheng and X. Lin, J. Synth. Cryst., 2012, 4, 468–473 Search PubMed.
- E. Okafor, J. Inorg. Nucl. Chem., 1980, 42, 1151–1159 CrossRef.
- S. Yu and Y. Li, Spectra Analytical Method, Chongqing University Press, Chongqing, 2nd edn, 1994 Search PubMed.
- X. Zhang, L. Lu, C. Bai, J. Liu and K. Yang, Rare Earth Luminescent Material, National Defence Industry Press, Beijing, 2005 Search PubMed.
- Y. Zhao, X. An, L. Wang, J. Bao and L. Yan, J. Chin. Rare Earth Soc., 2004, 2, 193–195 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |