Silver nanoparticle-embedded RGO-nanosponge for superior catalytic activity towards 4-nitrophenol reduction
Balakumar Vellaichamy and
Prakash Periakaruppan*
Department of Chemistry, Thiagarajar College, Madurai-625 009, Tamil Nadu, India. E-mail: kmpprakash@gmail.com; Fax: +91 4522312375; Tel: +91 9842993931
First published on 12th September 2016
Abstract
The present work highlights a bio-inspired synthesis of uniform 2 nm sized plasmonic silver nanospheres (Ag-NSs) embedded in reduced graphene oxide nanosponge (RGONS) using Tabebuia berteroi leaf extract. The green reduced RGONS/Ag-NSs is very clean and displays a fabulous catalytic activity towards the reduction of 4-nitrophenol (4-NP) in the presence of ice cold NaBH4 solution. Various analytical techniques were adopted to confirm the composition and structure of the crystalline nanocatalyst materials, including UV-visible absorption spectroscopy, FT-IR, XRD, Raman spectroscopy, HR-TEM and EDX. The formation of Ag-NSs, RGONS and RGONS/Ag-NSs, catalytic mechanism, stability and reusability of the catalyst were also investigated. The catalytic activity of RGONS/Ag-NSs is found to be superior to that ever reported.
Introduction
Metal nanoparticles (MNPs) have received tremendous interest due to their enormous applications in petrochemical industries and oil refineries since they catalyze various conversions such as C–C cross coupling, hydrogenation–dehydrogenations, electron transfers and hydrocarbon oxidations which are industrially important.1 In particular MNPs have attracted great attention as heterogeneous catalysts due to their interesting structure and high catalytic activities.2 It is well-known that nanosized metal particles have a high surface-to-volume ratio that can dramatically enhance the interaction between reactants and catalysts.3,4 Additionally, nanocatalysis can make the products easily removable from the reaction mixtures and make the catalysts recyclable. Moreover MNPs could act as a redox catalyst in the degradation of dyes by electron relay effect between donor and acceptor molecules.5 The catalytic properties of MNPs are appealing to many researchers because of their high reactivity and selectivity. Particularly silver nanoparticles (AgNPs) are of great interest both in fundamental research and industrial applications. AgNPs by virtue of their versatility, their unique features such as low toxicity, biocompatibility, catalytic activity, antimicrobial activity, optical and electrochemical properties have been extensively explored for various applications.5,6 Another quality of AgNPs is that it is the most accepted catalyst for the oxidation of ethylene to ethylene oxide and methanol to formaldehyde.7 However homogeneous nanocatalyst is undesirable for industrial and environmental applications because of their number of drawbacks, such as easy aggregation or precipitation, reduction in catalytic activities, difficulty in product separation and catalysts recycling. For this reason, it is highly desirable to design and synthesize heterogeneous catalyst with long-term stability, high surface to volume ratio, enhanced catalytic efficiency, high surface energy, and reusability.8–13 For achieving these features, MNPs are generally anchored on a variety of supporting substrates including carbon nanotubes,14 polymer,15 silica,16 alumina,17 titania,18 zirconia,19 and metal oxides20 to form composite catalysts, which have been verified to be an effective method to obtain high catalytic activity and stability. Amongst them, graphene supported NPs are emerging catalytic materials because of its extraordinary electronic transport, excellent optical and mechanical properties. Especially, these new carbonaceous supported materials exhibit large specific surface area, high charge carrier capacity and mobility, superior chemical stability and low production cost. Such unique features offer great promise for many potential applications.21–24 The AgNPs decorated graphene sheets can be synthesized by various methods. Though chemical synthesis has been widely adopted for the preparation of a variety of nanocatalyst, their cost effectiveness, stabilizing and capping agents and generation of hazardous organic substances to human health and the environmental hitches fade not only the applications of NPs but also the dream of a green world.25 Hence, there is an alarming demand for the synthesis of nanocatalyst, which is non-toxic, cost-effective and eco-friendly.26On the other hand, 4-nitrophenol (4-NP) is a refractory pollutant often present in many chemicals from drug industries and wastewater in outdoors as well as in the greenhouse systems worldwide. 4-NP is considered as a diesel exhaust particle and it has an adverse impact on both male and female reproductive functions. It also exhibits estrogen-like effects on female rats and anti-androgen-like effects on male rats. 4-NP is a well-known hazardous pollutant to cause damage to the central nervous system, liver, kidney, erythema, scaling, scabbing, cracking of the skin and causes headaches, drowsiness, nausea, cyanosis, eye irritation and infertility.27 Environmental contaminant such as 4-NP can adversely affect human reproduction and child development in a various ways resulting in impaired fertility, miscarriage or fetal death, altered fetal growth, birth defects and other developmental disorders, some of which may not become apparent for years.28 Infertility is a common problem, affecting perhaps one couple in six, the majority of whom now seek medical care. Although diagnostic problems make it difficult to establish the extent of the male partner's contribution with certainty, a number of studies suggest that male problems represent the commonest single defined cause of infertility.29 4-NP is the real culprit which cause major difficulties for accurate and meaningful diagnosis of male reproductive dysfunction that serves to complicate our understanding of the epidemiology and aetiology of male infertility. The World Health Organization has proposed a scheme for the diagnostic classification of male infertility, based upon a standardized approach to clinical assessment and to the assessment of semen quality.30 Consequently, US Environmental-Protection Agency has rated 4-NP as a priority pollutant and recommended to restrict its concentration in the natural water below 10 ng ml−1.31 Increasingly, the ill effect of 4-NP vis-à-vis the male infertility is being recognized now-a-days. Hence its removal from the environment is a crucial task. But 4-aminophenol (4-AP), the detoxificated form of 4-NP is an industrially important intermediate compound for pharmaceuticals, polymers, germicides, algaecides, fungicides, herbicides, dyes, wood protectors, explosives, plant growth regulators, analgesic and antipyretic drugs, photographic developers, corrosion inhibitors, anticorrosion lubricants and other fine chemicals.26,32–35
Herein we report the use of Tabebuia berteroi leaf extract (TBLE) as both reducing as well as stabilizing agent for the synthesis of RGONS, Ag-NSs and RGONS/Ag-NSs. The synthesized RGONS, Ag-NSs and RGONS/Ag-NSs were characterized by UV-visible spectroscopy, FT-IR spectroscopy, XRD, Raman spectroscopy, HR-TEM and EDX spectroscopy. The reaction rate and the surface plasmon resonance (SPR) of the formed Ag-NSs were continuously monitored by UV-visible spectroscopy. The synthesized RGONS, Ag-NSs and RGONS/Ag-NSs were used to detoxify 4-NP in the presence of ice-cold NaBH4 solution. The heterogeneous RGONS/Ag-NSs shows high catalytic activity compared to homogeneous RGONS and Ag-NSs. To date no work has been carried out on the catalytic depollution of 4-NP using synthesized RGONS/Ag-NSs.
Experimental section
The silver nitrate (AgNO3) was purchased from Sigma-Aldrich, India and used as received. The fresh Tabebuia berteroi (TB) leaf was collected in Thiagarajar College campus, Madurai, India. Raw graphite with average diameter about >20 μm was obtained from Sigma Aldrich. 4-NP, NaNO3, KMnO4, H2SO4, HCl, H2O2 and NaBH4 were purchased from Merck, India and used as received. All other chemicals were of analytical grade and used as such.Synthesis of GO
GO was synthesized using graphite powder by the modified Hummers method.36Preparation of TBLE
The fresh TB leaves were finely cut into small pieces and washed several times with deionized water to remove any dust and other contaminated organic contents. The 20 mg of small pieces of TB leaves was boiled with 10 mL of deionized water at 90 °C for 15 min and the extract was thrice filtered using Whatmann filter paper no. 1 to remove particulate matter and to get clear solution. The filtered TBLE was pale yellow in color and it was used as reducing as well as stabilizing agent for the synthesis of RGONS, Ag-NSs and RGONS/Ag-NSs. The filtrate solution was stored in the refrigerator at 4 °C for further experiments.Synthesis of crystalline Ag-NSs
In a typical experimental method, 50 mL of TBLE was mixed with 100 mL of aqueous solution of 1 mM AgNO3 at room temperature. The pale yellow color solution becomes deeper brown within an hour and no noticeable difference in the color of aqueous silver colloids is observed, which indicates that the bio-reduction process is over within an hour. The brown colour change indicates the formation of Ag-NSs in aqueous solution due to excitation of surface plasmon vibration in the metal nanoparticles. The synthesized Ag-NSs were collected by centrifugation and washed several times with deionized water. The dried Ag-NSs were lyophilized in ambient condition. After lyophilisation, the Ag-NSs were stored in a screw cap bottle for further characterization.Synthesis of RGONS
1 mg GO powder was dispersed in 5 ml of deionized water, which was subjected to ultrasonication for 30 min to give a stable transparent light brown suspension. Then, 5 mL of TBLE was added into the above GO suspension and the solution was mixed by ultrasonication for 30 min. The mixture was transferred into a Teflon-lined stainless steel autoclave and kept at 95 °C for 2 h. The resulting RGONS suspension was filtered and washed several times to remove any excess amount of organic moieties.Synthesis of RGONS/Ag-NSs
1 mg GO powder was dispersed in 5 ml of deionized water, which was ultrasonicated for 30 min to give a stable light brown suspension. 10 mL of TBLE and 5 mL of AgNO3 (0.001 mM) were added into GO suspension. The solution was ultrasonicated for 30 min. Then, it was transferred into a Teflon-lined stainless steel autoclave and kept at 95 °C for 2 h. The resulting RGONS-Ag nano suspension was filtered and washed several times to remove any excess amount of organic moieties. The resulting black precipitate was dried or re-dispersed in water for further characterization.Catalytic activity of RGONS, Ag-NSs and RGONS/Ag-NSs
In a typical experiment at room temperature, 1.8 mL of 4-NP (0.1 mM, aqueous solution) was mixed with 0.7 mL of NaBH4 (0.01 M aqueous solution) in a quartz cell (3.0 mL). Then, 0.005 mg of RGONS/Ag-NSs catalyst was added to the mixture of 4-NP and NaBH4 and the changes in the absorbance of the solution were monitored with a UV-visible spectrophotometer at different time intervals. After the reduction reaction was over, the mixture was centrifuged and washed with deionized water for several times. The resulting RGONS/Ag-NSs catalyst was reused in the next reaction. The homogeneous RGONS and Ag-NSs were also checked for their catalytic activity adopting the same procedure.Instrumental characterization
UV-visible spectra were measured using a Jasco (V-560) model UV-visible double beam spectrophotometer with 1 cm quartz cuvette. The FT-IR spectral data were recorded using KBr disc on a JASCO FT-IR 460 Plus spectrophotometer. XRD analysis was carried out in X-ray diffraction unit, Cu Kα radiation (λ = 1.5418 Å) on JEOL JDX 8030 X-ray diffractometer. Raman spectra were recorded on a Bruker senterra dispersive Raman microscope with laser excitation wavelength of 532 nm. The size and morphology of the Ag-NSs, RGONS and RGONS/Ag-NSs were examined by transmission electron microscopy (TEM, JEOL JEM 2100 model instrument).Energy dispersive X-ray (EDX) spectrometer attached to the transmission electron microscope was used for elemental analysis. All experiments were carried out at room temperature.
Results and discussion
The electronic spectroscopy is one of the simplest techniques to characterize the formation, size, shape and stability of the MNPs, because the MNPs exhibit strong absorption band due to surface plasmon resonance (SPR) in the visible region. Fig. 1a shows the absorption spectra recorded for TBLE in the presence and absence of AgNO3 solutions. TBLE shows two absorption peaks at 288 and 317 nm (curve a) corresponding to π–π* and n–π* transition of phenolic rings present in the TBLE. After addition of AgNO3 solution to TBLE solution, the pale yellow color becomes dark brown and a new absorption band at 423–437 nm (Fig. 1a (curve b–f)) is recorded at different time intervals (2, 10, 20, 30 and 1 h) corresponding to SPR. The absorption maxima of TBLE at 317 and 288 nm decrease simultaneously. After 1 h, the bands completely disappear. The observed SPR band at 437 nm confirms the formation of AgNSs.37 The absorption peak at 437 nm steadily increases and after 1 h there is no increase in the absorption peak (Fig. 1b), which confirms that the reaction is completed within 1 h. The intensity of color does not intensify after 1 h which is established by the plot as shown in Fig. 1c. Fig. 1d shows the UV-visible spectra of GO, RGONS and RGONS/Ag-NSs. The peaks appearing at 217 and 273 nm (curve a) may be attributed to the π–π* transition and n–π* transition of GO respectively. After reduction, the red shift of peak from 217 nm to 253 nm and the disappearance of peak at 273 nm (curve b), indicate that the electronic conjugation within the RGONS is revived upon reduction of GO.38 Also, the sharp absorption peaks appearing at 442 and 290 nm (curve c) confirm the formation of RGONS/Ag-NSs.39 Scheme 1 shows the detailed mechanism of the formation of Ag-NSs, RGONS and RGONS/Ag-NSs using TBLE and their catalytic activity towards the detoxification of 4-NP. | ||
| Scheme 1 Synthesis of the Ag-NSs, RGONS and RGONS/Ag-NSs using TBLE and their catalytic activity of 4-NP reduction. | ||
The dual role of the TBLE as a reducing and capping agent and the presence of various functional groups responsible for the reduction of silver nitrate to silver nanoparticles were confirmed by FT-IR analysis. The several characteristic peaks appearing in FT-IR spectra are shown in Fig. 2A (curve a). The existence of peak at 3402 cm−1 may be due to the –OH stretching vibration of alcohols and phenols or bending stretching of hydrogen-bonded alcohols and phenols in the leaf extract molecules. The peaks at 2932 and 2850 cm−1 are characteristics of stretching vibrations of methyl groups or C–H stretching vibrations of aldehydic amine groups. The peak at 1652 cm−1 is the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of carbonyl and carboxylic group of amide I and peaks at 1543 and 1253 cm−1 are the stretching vibrations of N–H groups and the bending vibrations of C–N groups, amide II and III bands, in the proteins.40 The peaks observed at 1456 and 1058 cm−1 are the bending vibrations of C–O–H groups and the anti-symmetric stretching band of C–O–C groups of polysaccharides and/or chlorophyll. The peak at 617 cm−1 is the plane bending vibration of N–H groups in the proteins.37,41 After the reduction of silver ions (Fig. 2A (curve b)), the peak intensity corresponding to –OH stretching vibration of phenols or high concentrated alcohols decreases and a shift is observed from 3402 to 3434 cm−1, illustrating the involvement of alcoholic groups in the nanospheres synthesis. The peak at 1652 cm−1 is due to C
O stretching vibration of carbonyl and carboxylic group of amide I and peaks at 1543 and 1253 cm−1 are the stretching vibrations of N–H groups and the bending vibrations of C–N groups, amide II and III bands, in the proteins.40 The peaks observed at 1456 and 1058 cm−1 are the bending vibrations of C–O–H groups and the anti-symmetric stretching band of C–O–C groups of polysaccharides and/or chlorophyll. The peak at 617 cm−1 is the plane bending vibration of N–H groups in the proteins.37,41 After the reduction of silver ions (Fig. 2A (curve b)), the peak intensity corresponding to –OH stretching vibration of phenols or high concentrated alcohols decreases and a shift is observed from 3402 to 3434 cm−1, illustrating the involvement of alcoholic groups in the nanospheres synthesis. The peak at 1652 cm−1 is due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of carbonyl and carboxylic groups in amide-I and the peak at 1543 cm−1 is due to N–H stretching vibration of amide-II linkage of proteins. After the encapsulation of silver ion, the peaks become weaker and are shifted, suggesting that the C
O stretching vibration of carbonyl and carboxylic groups in amide-I and the peak at 1543 cm−1 is due to N–H stretching vibration of amide-II linkage of proteins. After the encapsulation of silver ion, the peaks become weaker and are shifted, suggesting that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–H groups are also involving in the synthesis of nanospheres. The peak at 617 cm−1 completely disappears, which indicates that the reduction of the silver ions is coupled to the oxidation of the amine components.
O and N–H groups are also involving in the synthesis of nanospheres. The peak at 617 cm−1 completely disappears, which indicates that the reduction of the silver ions is coupled to the oxidation of the amine components.
 | ||
| Fig. 2 (A) FT-IR spectra of TBLE (curve a) and Ag-NSs (curve b) and (B) FT-IR spectra of GO (curve a), RGONS (curve b) and RGONS/Ag-NSs (curve c). | ||
Fig. 2B shows the FT-IR characteristic peaks of GO (curve a) at 3402, 1731, 1622, 1384, 1230, and 1058 cm−1, which are attributed to –OH stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of COOH, C–O stretching (epoxy or alkoxy), –OH deformation, C–OH stretching and C
O stretching of COOH, C–O stretching (epoxy or alkoxy), –OH deformation, C–OH stretching and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrations, respectively.42 After reduction, the C
C vibrations, respectively.42 After reduction, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band disappears and the peaks of other oxygenic functional groups in relation to GO strongly decrease. In FT-IR spectrum of RGONS (curve b), the peaks at 3429, 1637, and 1580 cm−1 are assigned to N–H stretching and C–H stretching in aromatic rings, amide I, and amide II, respectively. Further RGONS/Ag-NSs (curve c) peaks at 3432, 1630, and 1587 cm−1 are ascribed to N–H stretching and C–H stretching in aromatic rings, amide I, and amide II. The similarity of FT-IR spectra of the plant, RGONS, Ag-NSs and RGONS/Ag-NSs confirms that the constituents of extract are adsorbed on Ag-NPs surfaces by π-electrons interactions. Hence, the probable constituents of these phytochemicals (Fig. 3) present in the leaf extract may be responsible for the reduction and stabilization of RGONS/Ag-NSs.
O stretching band disappears and the peaks of other oxygenic functional groups in relation to GO strongly decrease. In FT-IR spectrum of RGONS (curve b), the peaks at 3429, 1637, and 1580 cm−1 are assigned to N–H stretching and C–H stretching in aromatic rings, amide I, and amide II, respectively. Further RGONS/Ag-NSs (curve c) peaks at 3432, 1630, and 1587 cm−1 are ascribed to N–H stretching and C–H stretching in aromatic rings, amide I, and amide II. The similarity of FT-IR spectra of the plant, RGONS, Ag-NSs and RGONS/Ag-NSs confirms that the constituents of extract are adsorbed on Ag-NPs surfaces by π-electrons interactions. Hence, the probable constituents of these phytochemicals (Fig. 3) present in the leaf extract may be responsible for the reduction and stabilization of RGONS/Ag-NSs.
The XRD strongly proves the formation of GO, RGONS, Ag-NSs and RGONS/Ag-NSs nanocatalyst. As reported earlier, the peak at 2θ = 13.01° corresponds to GO (Fig. 4a). After reduction with TBLE, the diffraction peak of GO at 2θ = 13.01° disappears and a broad peak around 2θ = 26.2° (Fig. 4b) is observed in the RGONS, indicating that most oxygen containing functional groups are removed.43 It should be noted that the diffraction peak in RGONS is broad and significantly different from GO.
The crystalline nature of Ag-NSs is confirmed by XRD analysis as shown in Fig. 4c. The four different diffraction peaks at 2θ values of 38.1°, 44.3°, 64.4° and 77.4° can be attributed to (1 1 1), (2 0 0), (2 2 0) and (3 1 1) crystallographic planes of face centered cubic (fcc) structure of metallic silver and is consistent with Joint Committee on Powder Diffraction Standards (JCPDS) data [no. 04-0783]. The sharp and strong signals of patterns evidence that the product are nanosized and well crystallized. Among the different planes, the peak corresponding to (1 1 1) plane is more intense than other planes suggesting it as a predominant orientation and the synthesized Ag-NSs are crystalline in nature. The width of the (1 1 1) peak was employed to calculate the average crystalline size of the Ag-NSs using Scherrer formula. The average size of the Ag-NSs is 7 nm, which precisely matches with the particle size obtained from the HR-TEM images.
Also the diffraction peaks are obtained at 2θ = 26.2°, corresponding to RGONS and peaks at 2θ = 38.1°, 44.4°, 64.8° and 77.6° corresponding to Ag-NSs. The XRD patterns confirm the formations of RGONS/Ag-NSs as shown in Fig. 4d.
Raman spectroscopy is one of the valuable spectra for the structural characteristics and properties of grapheme-based hybrid materials. Typical features of the GO in Raman spectra are the G line around 1600 cm−1 and the D line around 1350 cm−1, as shown in Fig. 5a. The G line is usually assigned to the first-order scattering of the E2g phonons of sp2 C atoms. The D line is the breathing mode of the j-point phonons of A1g symmetry.44 The D and G bands of the graphene are due to the breathing vibrations of carbon atoms of dangling bonds in plane termination of disordered and defected graphite and the in-plane bond-stretching vibration of sp2 bonded carbon atoms. The Raman spectra of the prepared GO, RGONS and RGONS-Ag-NSs (Fig. 5a–c) show two absorption bands (for D and G bands). Additionally, the presence of Ag-NSs in composites heightens the relative intensity ratio of D/G, which represents the degree of disorder. The intensity ratios are 0.85, 1.17 and 1.58 for GO, RGONS, and RGONS/Ag-NSs, respectively. The enlarged ratio for the RGONS/Ag-NSs sample confirms the enhanced disorderliness of the RGONS, owing to the interactions between the RGONS and Ag-NSs.
The surface morphology, size and shape of the Ag-NSs, RGONS and RGONS/Ag-NSs were identified using HR-TEM images. Fig. 6 shows the different magnifications of TEM images of the uniformly unagglomerated Ag-NSs with a size of 7 nm. Fig. 7a shows the particle size distribution (histogram) of the synthesized Ag-NSs. Fig. 7b shows the chemical analysis of the synthesized Ag-NSs by means of EDX which confirms the existence of Ag. The TEM results are well supported by all other experimental results of the formation of the synthesized Ag-NSs. Fig. 8a and d clearly shows the TEM images of RGONS and RGONS/Ag-NSs. Fig. 8a and b indicates that the synthesized RGO has nanosponge shape like structure on the surfaces. From Fig. 8c and d, it is clearly seen that the RGONS has been engrained with uniform Ag-NSs with a controlled size of 2 nm. The crystallinity of RGONS and RGONS/Ag-NSs is observed by selected area emission diffraction (SAED) pattern as shown in Fig. 9a and b. Fig. 9c shows a histogram indicating the particle size distribution of Ag-NSs engrained on RGONS and Fig. 8d shows the EDX pattern of RGONS/Ag-NSs which confirms the existence of C, O and Ag elements.
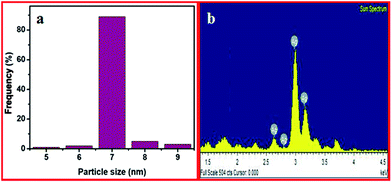 | ||
| Fig. 7 Histogram showing the particle size distribution of Ag-NSs (a) and EDX spectrum of the Ag-NSs (b). | ||
 | ||
| Fig. 9 SEAD patterns of RGONS (a), RGONS/Ag-NSs (b), a histogram showing the particle size distribution of Ag-NSs engrained on RGONS (c) and EDX spectrum of the RGONS/Ag-NSs (d). | ||
The catalytic activity of the as-prepared RGONS/Ag-NSs was evaluated through the model detoxification reaction of 4-NP to 4-AP using NaBH4 as the reducing agent and the catalytic conversion was easily monitored by UV-visible spectroscopy. The RGONS/Ag-NSs catalyst acts as an electronic relay system which involves electron transfer from donor BH4− to electron acceptor nitro group of 4-NP.45–58 An aqueous solution of 4-NP has a maximum absorption peak at 317 nm. After the addition of freshly prepared NaBH4 solution, the color of the solution changes from light yellow to dark yellow immediately and an absorption peak at 400 nm is observed due to the formation of 4-nitrophenolate ions in the reaction mixture as shown in Fig. 10a. 4-Nitrophenolate ion is more stable because the negative charge on the oxygen atom is effectively delocalized throughout the benzene ring and the reaction mixture becomes resonance stabilized.59
Without adding a catalyst, the color of the solution remains dark yellow and the absorption peak at 400 nm remains unaltered even for a couple of days (Fig. 10b), indicating that the reduction reaction does not take place. In the absence of catalyst, the thermodynamically favorable reduction of the nitro compound is not observed under the experimental condition. With the addition of homogenous catalysts such as RGONS and Ag-NSs, the reduction reaction is initiated by the decolorization of the 4-nitrophenolate solution which takes place in a couple of hours with RGONS and 20 minutes with Ag-NSs as shown in Fig. 10c and d. When RGONS/Ag-NSs catalyst (0.005 mg) is added into the solution, the reduction reaction is completed by the decolorization of the 4-nitrophenolate solution in just 120 s only. Further the absorption peak intensity at 400 nm decreases with a new adsorption peak at 295 nm corresponding to the formation of 4-AP as shown Fig. 10e. The identification of the catalytic property is made possible by 4-NP to 4-AP conversion without formation of any other by-products. The progress of the conversion can be conveniently measured by UV-visible spectroscopy leading to the rate constant k. The reaction kinetics can be described as −ln(C/C0) = kt, where k is the rate constant at a given temperature and t is the reaction time. C0 and C are the 4-NP concentration at the beginning and at time t, respectively. As expected, a good linear correlation of ln(C/C0) vs. reaction time t, up to 98% was obtained (Fig. 10f). The kinetic rate constant k is calculated from the slopes of the linear sections of the plots.
The value of k is calculated to be for 4-NP: 5.538 × 10−2 s−1 at room temperature. Fig. 11a illustrates the conversion percentage vs. time plots towards 4-NP to 4-AP reduction using RGONS/Ag-NSs as a catalyst. The RGONS/Ag-NSs exhibits higher catalytic activity compared to other earlier reported results which are summarized in Table 1.45–58 It is mentioned here that the Ag-NSs engrained RGONS in the solution are relatively smaller in size (2 nm), which contributes to the intrinsic electronic properties, high surface to volume ratio and these exposed atoms act strongly as a catalyst.
 | ||
| Fig. 11 Plot of the 4-NP conversion% vs. time (a) and plot of 4-NP conversion% vs. number of cycles. | ||
| Name of the catalyst | Catalyst (weight) | Size (nm) | Shape | Rate constant (k) | Conversion time | References |
|---|---|---|---|---|---|---|
| Ag–Fe3O4@chitin | 50 mg L−1 | 10–40 | Spherical | 0.3139 × 10−3 min−1 | 10 min | 45 |
| PANI/Ag | 1 mg | 50–70 | Spherical | 2.56 × 10−2 s−1 | 3 min | 46 |
| Ag/RGO | — | 40–45 | Nano-crystal | 0.3895 × 10−3 min−1 | 8 min | 47 |
| Ag/KCC | 0.2 mg | 200 | Spherical | 1 × 10−2 s−1 | 8 min | 48 |
| Fe3O4@P@Ag | 1.0 g | 20–25 | Spherical | 1.5285 × 10−3 min−1 | 0.17 min | 49 |
| Micron-SiO2@nano-Ag | 15 mg | 10–60 | Spherical | 3.56 × 10−3 s−1 | 12 min | 50 |
| Ag/TiO2–Cu | 0.05–0.2 g L−1 | 10 | Spherical | — | 25 min | 51 |
| Ag@SiO2 | — | 15 | Spherical | 0.37 min−1 | 12 min | 52 |
| Ag@AHs | — | 20 | Spherical | 0.243 min−1 | 11 min | 53 |
| Ag NPs/perlite | 5 mg | 8–25 | Spherical | — | 4 min | 54 |
| Ag@MWCNTs-polymer | 0.025 | 7–10 | Spherical | 7.88 × 10−3 s−1 | 5 min | 55 |
| Ag/PAN | 10 mg | 57 | Spherical | 0.012 min−1 | 50 min | 56 |
| MCF-n-Ag-m | 0.05–0.20 mg | 5–10 | Spherical | 2.66 × 10−2 s−1 | 5 min | 57 |
| Au–Ag | — | 5–20 | Spherical | 0.798 min−1 | 6 min | 58 |
| RGONS/Ag-NSs | 0.005 mg | 2 | Spherical | 5.35 × 10−2 s−1 | 2 min | Present work |
The turnover number (TON) and turnover frequency (TOF) are two important indicators to access the catalytic activity.59 TON was calculated by dividing the concentration of the 4-NP (mol) by the amount of the loading catalyst (weight (%)). TOF was calculated by dividing TON with the reaction time (h). The calculated TOF of RGONS/Ag-NSs is found to be 10 mmol mg−1 min−1, which suggests a high catalytic activity for RGONS/Ag-NSs.
Recyclability and stability are important characteristics of a good catalyst. In order to investigate the recyclability of the RGONS/Ag-NSs, the same catalyst was utilized repeatedly seven times for the detoxification reaction. After each use, the catalyst was centrifuged, washed and dried for the next cycle of catalysis. The catalyst exhibits high activity for the conversion even after seven cycles as shown in Fig. 11b.
The reaction of NaBH4 with graphene-based materials and its composition of AgNPs is an interesting area of research due to its potential application in aqueous environment. The mechanism of the catalytic reduction reaction of 4-NP to 4-AP in the presence of reducing agent and RGONS/Ag-NSs catalyst can be explained by Langmuir–Hinshelwood mechanism.60 NaBH4 is ionized in water to offer BH4−, which provides surface hydrogen for the reaction. The surface hydrogen is first transferred to RGONS/Ag-NSs, which enables the reduction of 4-NP to 4-AP as shown in Scheme 2. The RGONS/Ag-NSs acts as an electronic relay system wherein the electron transfers from the donor BH4− to the acceptor nitro groups. This reversible step can be modeled in terms of a Langmuir isotherm. The RGONS/Ag-NSs serves as a catalyst to provide active sites to promote the reaction.
 | ||
| Scheme 2 The reaction mechanism for the conversion of 4-NP catalyzed by the RGONS/Ag-NSs in the presence of NaBH4. | ||
FT-IR spectra and XRD patterns of RGONS/Ag-NSs after reduction reaction of 4-NP to 4-AP are shown in Fig. 12a and b, which indicates that the functional groups on the surface of the RGONS/Ag-NSs play no role in promoting the catalysis.
 | ||
| Fig. 12 (A) FT-IR spectra of RGONS/Ag-NSs – pre catalysis (a) and post catalysis (b) and (B) XRD patterns of RGONS/Ag-NSs – pre catalysis (a) and post catalysis (b). | ||
Conclusions
In summary, the RGONS, Ag-NSs and RGONS/Ag-NSs were synthesized by simple reduction approach. The smaller sized and uniformly well-dispersed plasmonic Ag-NSs engrained RGONS offers a good mechanical stability and large surface area of active sites for the higher catalytic activity of 4-NP reduction reaction. Graphene has been successfully employed as a support to stabilize and disperse the Ag-NSs. The synthesized RGONS/Ag-NSs catalyst exhibits a higher catalytic activity for the conversion even after seven cycles. In addition, the RGONS/Ag-NSs hybrid material exhibits improved catalytic activity compared to Ag-NSs synthesized without RGONS. The catalytic activity of RGONS/Ag-NSs is found to be superior to that ever reported. The catalyst of such kind proposed in this study can be used in wastewater treatment for the decontamination of 4-NP in aqueous solution.References
- M. Li and G. Chen, Nanoscale, 2013, 5, 11919–11927 RSC.
- Y. Sun and C. Lei, Angew. Chem., Int. Ed., 2009, 48, 6824–6827 CrossRef CAS PubMed.
- H. Lee, S. E. Habas, S. Kweskin, D. Butcher, G. A. Somorjai and P. Yang, Angew. Chem., Int. Ed., 2006, 45, 7824–7828 CrossRef CAS PubMed.
- M. D. Hughes, Y.-J. Xu, P. Jenkins, P. McMorn, P. Landon, D. I. Enache, A. F. Carley, G. A. Attard, G. J. Hutchings and F. King, Nature, 2005, 437, 1132–1135 CrossRef CAS PubMed.
- B. Wiley, Y. Sun and Y. Xia, Acc. Chem. Res., 2007, 40, 1067–1076 CrossRef CAS PubMed.
- M. Rycenga, C. M. Cobley, J. Zeng, W. Li, C. H. Moran, Q. Zhang, D. Qin and Y. Xia, Chem. Rev., 2011, 111, 3669–3712 CrossRef CAS PubMed.
- H. Wu, X. Huang, M. Gao, X. Liao and B. Shi, Green Chem., 2011, 13, 651–658 RSC.
- P. P. Zhang, X. X. Zhang, H. X. Sun, R. H. Liu, B. Wang and Y. H. Lin, Tetrahedron Lett., 2009, 50, 4455–4458 CrossRef CAS.
- Y. Lu, J. Yuan, F. Polzer, M. Drechsler and J. Preussners, ACS Nano, 2010, 4, 7078–7086 CrossRef CAS PubMed.
- Z. Li and H. C. Zeng, Chem. Mater., 2013, 25, 1761–1768 CrossRef CAS.
- X. Zhang and Z. Su, Adv. Mater., 2012, 24, 4574–4577 CrossRef CAS PubMed.
- M. H. Rashid and T. K. Mandal, Adv. Funct. Mater., 2008, 18, 2261–2271 CrossRef CAS.
- T. Premkumar and K. Geckeler, Colloids Surf., A, 2014, 456, 49–54 CrossRef CAS.
- X. Lu, X. Bian, G. Nie, C. Zhang, C. Wang and Y. Wei, J. Mater. Chem., 2012, 22, 12723–12730 RSC.
- X. C. Wang, L. Q. Meng, F. Wu, Y. J. Jiang, L. Wang and X. D. Mu, Green Chem., 2012, 14, 758–765 RSC.
- J. F. Pang, A. Q. Wang, M. Y. Zheng, Y. H. Zhang, Y. Q. Huang, X. W. Chen and T. Zhang, Green Chem., 2012, 14, 614–617 RSC.
- S. Schimpf, C. Louis and P. Claus, Appl. Catal., A, 2007, 318, 45–53 CrossRef CAS.
- A. Shrotri, A. Kanksale, J. N. Beltramini, H. Gurav and S. V. Chilukuri, Catal. Sci. Technol., 2012, 2, 1852–1858 CAS.
- E. van Ryneveld, A. S. Mahomed, P. S. van Heerden, M. J. Green and H. B. Friedrich, Green Chem., 2011, 13, 1819–1827 RSC.
- M. Banu, S. Sivasanker, T. M. Sankaranarayanan and P. Venuvanalingam, Catal. Commun., 2011, 12, 673–677 CrossRef CAS.
- S. Stankovich, D. A. Dikin, G. H. B. Dommett, K. M. Kohlhaas, E. J. Zimney, E. A. Stach, R. D. Piner, S.-B. T. Nguyen and R. S. Ruoff, Nature, 2006, 442, 282–286 CrossRef CAS PubMed.
- K. P. Loh, Q. Bao, G. Eda and M. Chhowalla, Nat. Chem., 2010, 2, 1015–1024 CrossRef CAS PubMed.
- A. A. Balandin, Nat. Mater., 2011, 10, 569–581 CrossRef CAS PubMed.
- M. J. Allen, V. C. Tung and R. B. Kaner, Chem. Rev., 2010, 110, 132–145 CrossRef CAS PubMed.
- A. J. Dahl, L. Bettye, S. Maddux and E. J. Hutchison, Chem. Rev., 2007, 107, 2228 CrossRef PubMed.
- Y.-T. Woo and D. Y. Lai, Aromatic Amino and Nitro–Amino Compounds and Their Halogenated Derivatives, Patty's Toxicology, Wiley, New York, 2001, pp. 1–96 Search PubMed.
- Agency for Toxic Substances and Disease Registry (ATSDR), Toxicological Profile for Nitrophenols (Draft), Public Health Service, U.S. Department of Health and Human Services, Atlanta, GA, 1990 Search PubMed.
- T. Woodruff, J. Schwartz and L. Giudice, J. Epidemiol. Community Health, 2010, 64, 307–310 CrossRef PubMed.
- D. Stewart Irvine, European Society for Human Reproduction & Embryology, 1998, vol. 13, pp. 33–44 Search PubMed.
- P. J. Rowe, F. H. Comhaire, T. B. Hargreave and H. J. Mellows, WHO Manual for the Standardized Investigation and Diagnosis of the Infertile Couple, Cambridge University Press, Cambridge, 1993 Search PubMed.
- K US-EPA, Water quality criteria, US Environmental Protection Agency, Washington, DC, 1976, http://www.water.epa.gov/scitech/swguidance/standards/criteria/current/upload/2009-01-13-criteria-redbook.pdf Search PubMed.
- S. C. Mitchell and R. H. Waring, Aminophenols, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, 2002 Search PubMed.
- R. S. Dowing, P. J. Kunkeler and H. van Bekkum, Catal. Today, 1997, 37, 121–130 CrossRef.
- J. Li, C. Liu and Y. Liu, J. Mater. Chem., 2012, 22, 8426–8430 RSC.
- S. Saha, A. Pal, S. Kundu, S. Basu and T. Pal, Langmuir, 2009, 26, 2885–2893 CrossRef PubMed.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339–1939 CrossRef CAS.
- A. Gangula, R. Podila, M. Ramakrishna, K. Lohith, J. Chelli and M. R. Apparao, Langmuir, 2011, 27, 15268–15274 CrossRef PubMed.
- D. Li, M. B. Muller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101–105 CrossRef CAS PubMed.
- S. Xu, L. Yong and P. Wu, ACS Appl. Mater. Interfaces, 2013, 5, 654–662 CAS.
- J. Kasturi, S. Veerapandian and N. Rajendran, Colloids Surf., B, 2009, 68, 55–60 CrossRef PubMed.
- R. Emmanuel, K. Chelladurai, S.-M. Chen, P. Selvakumar, S. Padmavathy and P. Prakash, J. Hazard. Mater., 2014, 279, 117–124 CrossRef CAS PubMed.
- L. Gao, W. Yue, S. Tao and L. Fan, Langmuir, 2013, 29, 957–1964 CrossRef CAS PubMed.
- M. J. McAllister, J.-L. Li, D. H. Adamson, H. C. Schniepp, A. A. Abdala and J. Liu, Chem. Mater., 2007, 19, 4396–4404 CrossRef CAS.
- K. N. Kudin, B. Ozbas, H. C. Schniepp, R. K. Prudhomme, I. A. Aksay and R. Car, Nano Lett., 2007, 8, 36–41 CrossRef PubMed.
- B. Duan, F. Liu, M. He and L. Zhang, Green Chem., 2014, 16, 2835–2845 RSC.
- B. Ma, M. Wang, D. Tian, Y. Pei and L. Yuan, RSC Adv., 2015, 5, 41639–41645 RSC.
- N. Meng, S. Zhang, Y. Zhou, W. Nie and P. Chen, RSC Adv., 2015, 5, 70968–70971 RSC.
- Z. Dong, X. Le, X. Li, W. Zhang, C. Dong and J. Ma, Appl. Catal., B, 2014, 158–159, 129–135 CrossRef CAS.
- W. Zhou, Y. Zhou, Y. Liang, X. Fengc and H. Zhou, RSC Adv., 2015, 5, 50505–50511 RSC.
- M. Wang, D. Tian, P. Tian and L. Yuan, Appl. Surf. Sci., 2013, 283, 389–395 CrossRef CAS.
- H. G. Agileo and V. R. Gonzalez, Chem. Eng. J., 2015, 261, 53–59 CrossRef.
- B. P. Bastakoti, S. Guragain, S. Yusab and K. Nakashima, RSC Adv., 2012, 2, 5938–5940 RSC.
- L. Ai and J. Jiang, Bioresour. Technol., 2013, 132, 374–377 CrossRef CAS PubMed.
- A. Rostami-Vartooni, M. Nasrollahzadeh and M. Alizadeh, J. Alloys Compd., 2016, 680, 309–314 CrossRef CAS.
- S. M. Alshehri, T. Almuqati, N. Almuqati, E. Al-Farraj, N. Alhokbany and T. Ahamad, Carbohydr. Polym., 2016, 151, 135–143 CrossRef CAS PubMed.
- S. Gao, Z. Zhang, K. Liu and B. Dong, Appl. Catal., B, 2016, 188, 245–252 CrossRef CAS.
- J. Gao, J. Xu, S. Wen, J. Hu and H. Liu, Microporous Mesoporous Mater., 2015, 207, 149–155 CrossRef CAS.
- M. M. Kumari, J. Jacob and D. Philip, Spectrochim. Acta, Part A, 2015, 137, 185–192 CrossRef PubMed.
- W. Ye, X. Shi, J. Su, Y. Chen, J. Fu, X. Zhao, F. Zhou, C. Wang and D. Xue, Appl. Catal., B, 2014, 160–161, 400–407 CrossRef CAS.
- S. Wunder, F. Polzer, Y. Lu, Y. Mei and M. Ballauff, J. Phys. Chem. C, 2010, 114, 8814–8820 CAS.
| This journal is © The Royal Society of Chemistry 2016 |







