Germanium-based multiphase material as a high-capacity and cycle-stable anode for lithium-ion batteries†
Abstract
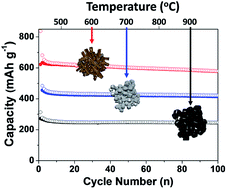
Copper germanate has been used in the electrical field and in lithium-ion battery anode applications. The known bonding energies between Cu and O enable us to control the chemical reduction process by thermal decomposition of hydro-carbon gas at high temperature. Herein, we demonstrate the synthesis of germanium-based multiphase materials by a carbothermic reduction process, in which the copper germanate (CuGeO3) single phase material is transformed to multiphase composite materials including Cu, Cu3Ge, GeOx, and Ge by controlling the reaction temperatures. The resulting Ge-based anodes exhibit a reversible capacity of ∼600 mA h g−1, stable capacity retention (80% after 100 cycles at 25 °C and 78% after 200 cycles at 60 °C) at a rate of C/5 with low electrode swelling (23% after 100 cycles). This is a one-step reaction process simultaneously involving reduction, phase transformation, and carbon coating. During the thermolysis reaction, various phases in the material are formed at the interface between Cu and Ge, which can affect the electrochemical performance of the resulting Ge-based anodes.

 Please wait while we load your content...
Please wait while we load your content...