Hydrate formation in water-laden microcapsules for temperature-sensitive release of encapsulants†
Juwoon Park‡a,
Sang Seok Lee‡
b,
Young Hoon Sohna,
Shin-Hyun Kim*b and
Yutaek Seo*a
aDepartment of Naval Architecture and Ocean Engineering, RIMSE, College of Engineering, Seoul National University, 1, Gwanak-ro, Gwanak-gu, Seoul 151-744, Korea. E-mail: yutaek.seo@snu.ac.kr
bDepartment of Chemical and Biomolecular Engineering (BK21+Program), Korea Advanced Institute of Science and Technology (KAIST), 291 Daehak-ro, Yuseong-gu, Daejeon 305-701, Republic of Korea. E-mail: kim.sh@kaist.ac.kr
First published on 30th August 2016
Abstract
Microcapsules have been widely used to store and release active materials for various purposes. In this work, we design microcapsules that separate an inner water phase from guest molecules in the surrounding medium with a polymeric shell. The water and guest molecules are brought into contact within the shell, where a hydrate is formed when the temperature is lower than the hydrate formation condition. A steady supply of water and guest molecules through the shell matrix into the hydrates yields local cracks in the shell. As the hydrates continue to grow in the absence of external shear flow, the cracks slowly propagate along the whole shell. In contrast, in the presence of external shear, the cracks formed by the hydrate formation are rapidly widened by the shear. This is the first direct evidence presenting the effects of hydrate formation on water-laden microcapsules. We believe that the microcapsules can be further engineered to produce temperature-sensitive microcarriers for controlled delivery of specialty chemicals.
Introduction
Microcapsules are typically composed of a liquid core and a solid shell which acts as a membrane separating the inner core from the outer environment. Microcapsules have been used for the long-term storage of active materials without leakage.1,2 In addition, their membranes can be designed to be responsive to controllable external stimuli such as light, magnetic fields, ultrasound, and electric fields. Therefore, the microcapsules can release specialty chemicals on-demand.3–5 If the membrane is responsive to environmental conditions such as temperature, pressure, and/or pH, the microcapsules sense the condition and selectively release the encapsulants in the absence of external triggering.4,5 Both on-demand and smart release functions are beneficial for a wide range of applications, including drug delivery. Recently, such microcapsules with advanced functions have been prepared using multiple-emulsion-based microfluidic technologies which provide high flexibility in material selection and high uniformity in size and composition of microcapsules.6,7 Most previous works have been focused on the engineering of shell property and the stimuli-responsive release under atmospheric pressure conditions. There is no study that reports how phase changes, such as the formation of gas hydrates, influence the solid shell and encapsulant release under pressurized conditions.Gas hydrates are non-stoichiometric crystalline compounds which are formed by enclathrating gas molecules with hydrogen-bonded water molecules under high pressure and relatively low temperature conditions.8 The gas molecules are called the guest in the host lattice structure formed by the water molecules and possible guest molecules include methane, ethane, and propane and liquid hydrocarbons such as cyclopentane and tetrahydrofurane. The formation of gas hydrates has been widely studied for gas storage,9–12 gas separation,13–15 and numerous other applications.16,17 Their formation process is generally dictated by the mass transfer of guest molecules into growing hydrate crystals. When a water drop shares an interface with bulk guest gases or hydrocarbon liquids, a hydrate is rapidly formed at the free interface. The hydrate layer formed along the interface then serves as a diffusion barrier, which slows down the growth of the hydrate. To control the hydrate formation process, the interface between host and guest has been engineered. For example, water adsorbed on silica gels, exposed to a guest gas phase, shows fast hydrate formation due to its large interfacial area; nanopores in the silica gel are partially coated with water molecules by adsorption, which are then fully filled with guest gas. Using the silica gel, carbon dioxide can be rapidly captured from pre-combustion gas and flue gas.13–15 Dry water, i.e. water droplets whose interface is stabilized by a multilayer of hydrophobic silica nanoparticles, also shows faster hydrate formation in comparison with the bulk water due to wide surface area.18–20 If the water droplets are separated from the hydrocarbon molecules by a solid membrane whose pores are comparable to or slightly larger than the molecules, the hydrate formation occurs through the sold membrane, which affects the mechanical properties of the membrane. To the best of our knowledge, there has been no systematic study on hydrate formation on such an engineered polymeric shell of microcapsules.
In this study, we prepare microcapsules composed of a water core and polymeric shell using a microfluidic technique and investigate the effects of hydrate formation on the stability of microcapsules. The polymeric shell has a densely cross-linked network structure which interrupts mass transfer, allowing limited rates of diffusion for water and guest molecules. When the microcapsules containing water, exposed to the guest molecules methane and cyclopentane, are subjected to low temperature and high pressure, a hydrate is formed as water and the guest molecules are brought into contact in the shell. Upon hydrate formation, the tough polymeric shell becomes fractured. Moreover, the microcapsules are torn apart under shear stress in the presence of a hydrate layer on the surface. This shell fracturing entails the release of encapsulants from the microcapsules to the surrounding. We attribute the crack formation to the crystal growth in the narrow free volume of cross-linked network in the shell. The high driving force to form a gas hydrate overwhelms the ultimate strength of the network at the microscopic level, resulting in the shell fracturing. The hydrate-induced release of encapsulants is potentially useful for many applications. For example, the microcapsules can be used as smart carriers for hydrate inhibitors. If the inhibitors are released at the initial stage of hydrate formation, we can avoid a plugging in subsea flowlines which is hazardous and costly problem for offshore fields.
Results and discussion
Microfluidic production of microcapsules containing water
We produce microcapsules composed of a water core and a polymeric shell and suspend them in a liquid hydrocarbon phase. The microcapsules are prepared by using water-in-oil-in-water (W/O/W) double-emulsion drops as a template. To produce the double-emulsion drops in a controlled manner, we use a capillary microfluidic device which is comprised of two tapered cylindrical capillaries assembled in a square capillary, as shown in Fig. 1a.21–24 The cylindrical capillary with an orifice diameter of 320 μm is treated to be hydrophobic and the other capillary with an orifice diameter of 480 μm is treated to be hydrophilic, they are then coaxially aligned within the square capillary to have a tip-to-tip distance of 260 μm. We inject an innermost water phase, a 2 w/w% aqueous solution of poly vinyl alcohol (PVA), through the hydrophobic cylindrical capillary. The red-colored dye disodium 2-hydroxy-1-(2-methoxy-5-methyl-4-sulfonatophenyl azo) naphthalene-6-sulfonate is additionally dissolved in the innermost water phase at a concentration of 0.14 w/w% to inspect leakage from the microcapsules. At the same time, the middle oil phase consisting of ethoxylated trimethylolpropane triacrylate (ETPTA) containing 1 w/w% photoinitiator is injected through the interstices between the hydrophobic cylindrical capillary and the square capillary. The continuous phase, a 10 w/w% aqueous solution of PVA, is injected through the interstices between the hydrophilic cylindrical capillary and the square capillary as a counter-flow to the innermost and middle phases. Volumetric flow rates of the inner, middle, and continuous phases are set to 2500, 1100, 4000 μL h−1, respectively. The middle phase wets the outer wall of the hydrophobic capillary, thereby facilitating the generation of water-in-oil drops at the tip. The interface between the middle oil and the continuous water phases is formed in between the two cylindrical capillaries, which breakups into oil drop at the moment of insertion of the innermost water drop, thereby producing W/O/W double-emulsion drops.25 This inner drop-triggered breakup of oil and formation of double-emulsion drops are shown in Fig. 1b and Movie S1 of the ESI.†The double-emulsion drops flow along the hydrophilic cylindrical capillary without adhesion on the wall as the continuous phase wets the entire surface of the capillary. The double-emulsion drops are then collected in a glass vial containing a 4 w/w% aqueous solution of PVA under continuous irradiation of UV light. The double-emulsion drops slowly sediment in the vial because their average density is slightly larger than that of the collection liquid. During the sedimentation, the middle layer of the double-emulsion drops is solidified into a polymeric shell through the photopolymerization of ETPTA.26 The resulting microcapsules are shown in Fig. 1c. More than 95% of microcapsules retain water without any leakage as confirmed by the red dye in their core. The microcapsules are highly monodisperse. Average radii of the inner cores and outer shells are 190 μm and 214.5 μm as shown in Fig. 1d and their coefficients of variation (CVs) are 3.99% and 3.62% respectively. That is, a membrane made of polymerized ETPTA (pETPTA) with average thickness of 24.5 μm is prepared. The production throughput of microcapsules is 3.6 g h−1 which is equivalent to 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 capsules per h.
250 capsules per h.
Hydrate formation on microcapsules and its Raman analysis
The water-laden microcapsules suspended in water are transferred into cyclopentane. During the phase transfer from water to the liquid hydrocarbon, multiple washing steps with cyclopentane are carried out. The microcapsules maintain their spherical water cores which are separated from the surrounding cyclopentane by intact solid shells. To investigate the hydrate formation on the microcapsules and to analyse the crystalline structure of the hydrate using Raman spectra, we deposit the microcapsules on a glass slide and leave an only small amount of cyclopentane. Most of remaining cyclopentane is positioned between the microcapsules and the glass slide, resulting in a thin film of cyclopentane covering the surface of the microcapsules, as illustrated in Fig. 2a. The microcapsules are then surrounded by pressurized methane in a high-pressure cell at 120 bar. The cell is incubated in a water bath at 1 °C for a week, which is much lower than the hydrate formation temperature of methane and cyclopentane: 31.8 °C at 121.9 bar. As soon as it is recovered from the cell, the microcapsules-on-glass slide is stored in liquid nitrogen to prevent hydrate dissociation in atmospheric pressure for Raman analysis.Over the course of heating from 93 K to 273 K, optical images and Raman spectra are acquired from the outer surface of microcapsule, as shown in Fig. 2b and c. At temperatures lower than 153 K, the surface is uneven due to frozen cyclopentane, as shown in the first and second panels of Fig. 2b. The frozen cyclopentane disappears as the temperature rises to 213 K, as shown in the third panel; the melting temperature of cyclopentane is 179.2 K. At a temperature of 213 K, fine grains of hydrate crystals are observed on the surface of the microcapsule. The hydrate crystals also disappear as the temperature is further increased to 273 K as shown in the last panel. Raman spectra measured over the course of heating reveal the crystalline structure of the hydrate. The Raman peaks at 2902 cm−1 and 2912 cm−1 indicate C–H stretching vibrations of methane encaged in large (51264) and small cages (512) of structure II hydrates, respectively. The more pronounced peak at 2912 cm−1 compared to the peak at 2902 cm−1 implies that most methane in the hydrate is captured in small cages. The Raman peak at 2868 cm−1 indicates C–H stretching vibrations of cyclopentane in large cage of structure II. A structure II hydrate composed of small and large cages that accommodate methane and cyclopentane is shown in Fig. 2d. The obtained Raman spectra confirm the formation of a methane and cyclopentane mixed hydrate on the surface of microcapsule. The formation of structure II hydrate for cyclopentane in the presence of methane is also consistent with other works.27 When the microcapsules are recovered at room temperature, a leakage of red-coloured dye is observed although the microcapsules retain their spherical shape.
Fracturing of microcapsule shells during hydrate formation
The pETPTA shell allows diffusion of water,28,29 thereby providing a water source for hydrate formation. However, it is still unknown how the hydrate formation influences the properties of polymeric shell. To clarify this, we directly observe the formation of hydrate in the microcapsules using an autoclave cell equipped with a visual window, as illustrated in Fig. 3a. Hydrate formation proceeds at 1 °C.The water-laden microcapsules in air have spherical, intact shells, as shown in Fig. 3c. Microcapsules incubated at 1 °C for 1 day have a hydrate layer on the surface of their polymeric shell, while retaining their red-dye-dissolved water core, as shown in top panel of Fig. 3d. The preservation of a large portion of the water core even after 1 day is attributed to the low permeability of the polymeric shell to water and gas molecules. The shells of microcapsules recovered after 1 day of incubation are locally cracked, while maintaining overall membrane integrity, as shown in bottom panel of Fig. 3d and S1a of the ESI.† Only small cracks are formed at the thin parts of the shells. Microcapsules that are subjected to incubation for 2 weeks at the same pressure and temperature conditions lose most of their inner core as shown in top panel of Fig. 3e. The shells are torn forming large gaps and some of them are broken into two or more pieces, as shown in bottom panel of Fig. 3e and S1b.† We attribute the crack formation and its widening to hydrate growth within the polymeric shell. The gas and water molecules can diffuse through the crosslinked network of the polymeric shell. Therefore, the host and guest are brought into contact in the middle of the shell, where the hydrate is nucleated and further grown. The hydrate growth stresses out the tough shell, making the crack, as illustrated in Fig. 3b. The cracks are expected to be formed at the thin part of the shell which has low depth of diffusion as well as a low critical stress for fracturing; this is consistent with observations for the 1 day incubation. As the hydrates grow, the cracks are widened, which propagate toward the thick part of the shell. Some of the cracks can form closed loops, which breaks the shell into two or more pieces, consistent with observations for the 2 weeks incubation. As the cracks are formed and widened, the gap is filled with hydrate. Therefore, encapsulants are slowly released from the core through the voids between the hydrate and cracked shell.
To confirm that the cracks are formed during hydrate formation rather than dissociation, the temperature is slowly increased to 20 °C for 5 days. The dissociation involves the release of gas from hydrate cages and sudden dissociation may cause sudden volume expansion, which could have a decisive effect on shell fracturing. The fraction of ruptured microcapsules at slow dissociation is comparable with that at fast dissociation for 1 hour as shown in Fig. S2 of the ESI,† inferring insignificant effect of dissociation rate on shell fracturing.
Influence of shear flow on the microcapsules
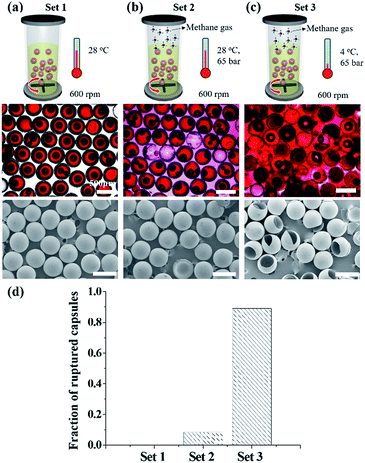
External shear flow can influence the response of microcapsules during hydrate formation. We study the influence with an autoclave equipped with a stirrer. In the autoclave, a suspension of water-laden microcapsules in cyclopentane is loaded with a volume ratio of water to cyclopentane of 40%, where no free water is present in the continuous phase. The microcapsule and cyclopentane mixture is stirred at 600 rpm; this condition corresponds to a Reynolds number of 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, implying turbulent mixing. At 28 °C and atmospheric pressure, most microcapsules survive without any cracking or leaking after 13 hours of mixing, as shown in Fig. 4a; air bubbles trapped in the microcapsules are formed during phase transfer from water to cyclopentane before mixing. The shear stress exerted by vigorous mixing is not able to damage the microcapsules with a highly crosslinked pETPTA shell. The methane gas is then applied at 65 bar, while maintaining the temperature at 28 °C and stirring at 600 rpm. Even with the high pressure, most microcapsules retain their water core and their shells remain intact with little damage, as shown in Fig. 4b; 8% of the microcapsules are ruptured, as shown in Fig. 4d. When the temperature is lowered to 4 °C, while maintaining the gas pressure and stirring, most microcapsules are ruptured, leaking water from the core as shown in Fig. 4c. More than 89% of the microcapsules are ruptured, as shown in Fig. 4d, which is much higher than almost 0% for stirring only (Fig. 4a) and 8% for stirring and gas pressure (Fig. 4b), indicating that the fracturing is triggered by the hydrate formation. The majority of ruptured microcapsules are broken into two or more pieces. This is in contrast to the microcapsules incubated in a static environment for 1 day in Fig. 3d. We attribute the intense fracturing to the shear stress of mixing. The hydrate formation in the shell causes cracks in the same manner to static experiment. In the presence of shear flow, the crack is rapidly widened by the random shear force exerted by turbulent mixing.
000, implying turbulent mixing. At 28 °C and atmospheric pressure, most microcapsules survive without any cracking or leaking after 13 hours of mixing, as shown in Fig. 4a; air bubbles trapped in the microcapsules are formed during phase transfer from water to cyclopentane before mixing. The shear stress exerted by vigorous mixing is not able to damage the microcapsules with a highly crosslinked pETPTA shell. The methane gas is then applied at 65 bar, while maintaining the temperature at 28 °C and stirring at 600 rpm. Even with the high pressure, most microcapsules retain their water core and their shells remain intact with little damage, as shown in Fig. 4b; 8% of the microcapsules are ruptured, as shown in Fig. 4d. When the temperature is lowered to 4 °C, while maintaining the gas pressure and stirring, most microcapsules are ruptured, leaking water from the core as shown in Fig. 4c. More than 89% of the microcapsules are ruptured, as shown in Fig. 4d, which is much higher than almost 0% for stirring only (Fig. 4a) and 8% for stirring and gas pressure (Fig. 4b), indicating that the fracturing is triggered by the hydrate formation. The majority of ruptured microcapsules are broken into two or more pieces. This is in contrast to the microcapsules incubated in a static environment for 1 day in Fig. 3d. We attribute the intense fracturing to the shear stress of mixing. The hydrate formation in the shell causes cracks in the same manner to static experiment. In the presence of shear flow, the crack is rapidly widened by the random shear force exerted by turbulent mixing.
Release of encapsulants upon hydrate formation
The unique behaviour of hydrate formation in the microcapsule system—hydrate formation and shell rupture—is expected to be beneficial for designing smart microcarriers to release active encapsulants at low temperature and high pressure. Upon hydrate formation, the polymeric shell is ruptured and the inner content is released to surroundings. Fig. 5 shows the release of encapsulants due to hydrate formation. The microcapsules containing red dye inside are safely suspended in cyclopentane without leakage as shown in Fig. 5a. When the temperature and pressure conditions fall into the hydrate formation zone, the red dye is released from the microcapsules to the surroundings as shown in Fig. 5b, where microcapsules covered by a hydrate layer are mixed with released red dye. The retrieved contents from the cell have an aqueous solution of the red dye at the bottom and cyclopentane at the top, as shown in Fig. 5c; fractured microcapsules precipitate at the bottom or attach to the side wall. This results clearly suggests that the microcapsules selectively release encapsulants only if the conditions are suitable for hydrate formation.The hydrate forming guest used in this work, cyclopentane, is able to form hydrates at about 9 °C under atmospheric pressure, and the formation temperature increases with the methane pressure. The hydrate formation temperature depends on the guest molecules; for example, tetrahydrofurane (THF) forms a hydrate at 4.3 °C and tetra-n-butylammonium bromide (TBAB) forms at 12.5 °C under atmospheric pressure.30 Therefore, it is possible to control the temperature range for encapsulant release by adjusting the pressure and by replacing guest molecules. The hydrate formation rate may affect the fracturing and release and more comprehensive works must be carried out in the near future. We believe that smart release triggered by hydrate formation will expand the application of microcapsules to a wide range of industrial process where the operation is sensitive to temperature change in pressurized condition.
Experimental
Materials
The cyclopentane was purchased from Sigma-Aldrich. The pure methane gas (99.95 mol%) and ethylene gas (99.99 mol%) were supplied by Special Gas (Korea). The distilled water was purchased from OCI (Korea) and used without further purification. As innermost and continuous phases, 2 w/w% and 10 w/w% aqueous solutions of PVA (Mw 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–23
000–23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Sigma-Aldrich) are used. As a photocurable middle phase, ETPTA (Mn 428, Sigma-Aldrich) containing 1 w/w% photoinitiator (Irgacure 2100, Ciba) is used.
000, Sigma-Aldrich) are used. As a photocurable middle phase, ETPTA (Mn 428, Sigma-Aldrich) containing 1 w/w% photoinitiator (Irgacure 2100, Ciba) is used.
Preparation of microcapsules
The water-in-oil-in-water (W/O/W) double emulsion droplets were generated by a glass capillary microfluidic device. Two cylindrical capillaries (1B100F-6, World Precision Instruments, Inc.) were tapered by a micropipette puller (P97, Sutter Instrument). One of them was sanded to have a 320 μm-orifice and treated with octadecyltrimethoxysilane (Sigma-Aldrich) to render it hydrophobic and the other was sanded to have 480 μm-orifice and treated with 2-[methoxy (polyethyeneoxy) propyl] trimethoxysilane (Gelest, Inc) to render it hydrophilic. These two cylindrical capillaries were coaxially aligned within a square capillary (OD 1.5 mm, ID 1.05 mm, Atlantic International Technologies, Inc.) with a 260 μm distance. The innermost aqueous solution was injected through the cylindrical capillary with a 320 μm-orifice, and the shell phase was injected through the interstice between the cylindrical and square capillaries. The continuous phase was injected through the interstice between the cylindrical capillary with a 480 μm-orifice and the square capillary. The typical volumetric flow rates of the three fluids from the innermost phase were controlled to be 2500, 1100, and 4000 μL h−1 by syringe pumps (Legato 100, KD scientific). The generation of double-emulsion drops was observed with an optical microscope (TS100, Nikon) equipped with a high speed camera (Motionscope M3, Redlake). The double-emulsion drops were collected in a 4 w/w% aqueous solution of PVA. To polymerize the shell phase, double-emulsion drops were continuously irradiated by fiber-coupled spot UV (Innocure 100N, Lichtzen Co.) during the collection in a vial. Average radii of inner core and outer shell are measured from optical microscope images, where more than 150 microcapsules are analysed. The polymerized microcapsules were transferred from water to cyclopentane through several washing step using a solution of cyclopentane with 2 wt% of Span 80 (Sigma-Aldrich) followed by redispersion in pure cyclopentane.Hydrate formation in static conditions
To analyse the structure of hydrates with Raman spectroscopy, 2 g of water-encapsulated pETPTA microcapsules dispersed in cyclopentane were placed into the high-pressure cell. The cell was pressurized with 120 bar methane gas at 24 °C, which was then immersed in a 1 °C water bath (Jeio Tech, RW-2025G) for a week to form hydrate. The sample was recovered and stored in liquid nitrogen to prevent the hydrate dissociation into the atmosphere for Raman analysis.To observe the hydrate formation, 2 g of water-encapsulated microcapsules were transferred into air by removing all the cyclopentane, and were then placed in the autoclave cell (316 SUS with 50 bar of maximum working pressure) equipped with a visual window. The cell was pressurized with ethylene gas to 30 bar, which was maintained at 1 °C in the water bath. The microcapsules in the cell were observed by optical microscope after 1 day and 2 weeks. The microcapsules recovered at room temperature were observed by SEM.
Hydrate formation in dynamic conditions
To study the influence of shear flow, water-encapsulated microcapsules dispersed in cyclopentane were placed in a 100 mL high-pressure cell (316 SUS) equipped with an anchor-type impeller. The amount of water encapsulated in the 30 mL of total liquid phase is set to be 40% (water cut). Methane gas was charged in the cell to have a pressure of 65 bar at 28 °C, while agitating the liquid phase at 600 rpm for 2 h to saturate the cyclopentane with methane. The cell was cooled from 28 °C to 4 °C by a constant cooling method with a 0.25 K min−1 rate under isochoric conditions and maintained at these conditions for an additional 10 h. The fractions of ruptured microcapsules are determined from optical microscope images of more than 150 microcapsules; the ruptured microcapsules lose pigments, rendering them easily distinguishable from intact ones.Characterization
An optical microscope (Ti, Nikon) equipped with a camera (DS-Ri1, Nikon) and scanning electron microscope (SEM, S-4800, Hitachi) were used to observe the shell of the microcapsules. An optical microscope (L150, Nikon) equipped with a camera (DS-5M, Nikon) was used to observe the formation of hydrate on the membrane. The Raman spectra were recorded using a Horiba Jobin-Yvon ARAMIS high resolution dispersive Raman microscope equipped with an electrically cooled (203 K) CCD detector. The excitation source was an Ar-ion laser emitting a 514.53 nm line, and the laser intensity was 30 mW. The experimental temperature was changed from 93 K to 243 K by a Linkam TMS 94 temperature controller with liquid nitrogen during the measurement.Conclusions
In this work, we investigate the hydrate formation in a microcapsule system. Water is completely enclosed by the permeable shell of the microcapsules, separated from a gas or oil environment. The shells crack upon hydrate formation as the hydrates are nucleated and grown within the shell where the molecules are brought into contact. Under static conditions, the cracks are formed in the thin part of shell, which then slowly propagate as the hydrate is further grown at the gaps of the cracks. By contrast, under dynamic, shear flow conditions, the cracks are formed along the shell and then rapidly widened by the shear stress. As we confirmed, the rupturing of the polymeric shell is triggered by the hydrate formation, thus the microcapsules can be designed to release their inner contents only when they experience the conditions responsible for the hydrate formation. The microcapsules can be further engineered to produce smart microcarriers for the environment-responsive release of specialty chemicals. At the same time, as the microfluidic system has a limited throughput of production, bulk synthetic methods are required to be developed for practical uses. The hydrate formation and accompanied release will expand the application of microcapsules to a wide range of industries where operations are sensitive to temperature change.Acknowledgements
This work was supported by the Industrial Strategic Technology Development Program (No. 10045068) of the Korea Evaluation Institute of Industrial Technology (KEIT) funded by MOTIE and the Research and Development Program of the Korea Institute of Energy Research (KIER; B4-2434-01). This work was also partially supported by the Technology Innovation Program (No. 10060099) funded by the Ministry of Trade, Industry & Energy (MI, Korea).Notes and references
- B. Comiskey, J. D. Albert, H. Yoshizawa and J. Jacobson, Nature, 1998, 394, 253–255 CrossRef CAS.
- S.-H. Kim, S.-J. Jeon and S.-Y. Yang, J. Am. Chem. Soc., 2008, 130, 6040–6046 CrossRef CAS PubMed.
- S. J. Pastine, D. Okawa, A. Zettl and J. M. J. Frechet, J. Am. Chem. Soc., 2009, 131, 13586–13587 CrossRef CAS PubMed.
- A. P. Esser-Kahn, S. A. Odom, N. R. Scottos, S. R. White and J. S. Moore, Macromolecules, 2011, 44, 5539–5553 CrossRef CAS.
- T. S. Shim, S.-H. Kim and S.-M. Yang, Part. Part. Syst. Charact., 2013, 30, 9–45 CrossRef CAS.
- W. J. Duncanson, T. Lin, A. R. Abate, S. Seiffert, R. K. Shah and D. A. Weitz, Lab Chip, 2012, 12, 2135–2145 RSC.
- T. Y. Lee, T. M. Choi, T. S. Shim, R. A. M. Frijns and S.-H. Kim, Lab Chip, 2016, 16, 3415–3440 RSC.
- E. D. Sloan and C. A. Koh, Clathrate Hydrates of Natural Gases, CRC Press, Taylor & Francis Group, Boca Raton, FL, 3rd edn, 2008 Search PubMed.
- H. Lee, J. W. Lee, D. Y. Kim, J. Park, Y. T. Seo, H. Zeng, I. L. Moudrakovski, C. I. Ratcliffe and J. A. Ripmeester, Nature, 2005, 434, 743–746 CrossRef CAS PubMed.
- J. Park, K. Shin, J. W. Lee, H. Lee and Y. Seo, Can. J. Chem., 2015, 93, 1035–1042 CrossRef CAS.
- D. Sun and P. Englezos, Int. J. Greenhouse Gas Control, 2014, 25, 1–8 CrossRef CAS.
- R. Kumar, P. Linga, I. Moudrakovski, J. A. Ripmeester and P. Englezos, AIChE J., 2008, 54, 2132–2144 CrossRef CAS.
- Y. Seo and S. P. Kang, Chem. Eng. J., 2010, 161, 308–312 CrossRef CAS.
- S. P. Kang, J. Lee and Y. Seo, Chem. Eng. J., 2013, 218, 126–132 CrossRef CAS.
- S. P. Kang and H. Lee, Environ. Sci. Technol., 2000, 34, 4397–4400 CrossRef CAS.
- W. Lee, M. Kwon, S. Park, D. Lim, J. H. Cha and H. Lee, Chem.–Asian J., 2013, 8, 1569–1573 CrossRef CAS PubMed.
- J.-H. Cha, W. Lee and H. Lee, Angew. Chem., Int. Ed., 2009, 48, 8687–8690 CrossRef CAS PubMed.
- W. Wang, C. L. Bray, D. J. Adams and A. I. Cooper, J. Am. Chem. Soc., 2008, 130, 11608–11609 CrossRef CAS PubMed.
- B. O. Carter, W. Wang, D. J. Adams and A. I. Cooper, Langmuir, 2010, 26, 3186–3193 CrossRef CAS PubMed.
- J. Park, K. Shin, J. Kim, H. Lee, Y. Seo, N. Maeda, W. Tian and C. D. Wood, J. Phys. Chem. C, 2015, 119, 1690–1699 CAS.
- A. S. Utada, E. Lorenceau, D. R. Link, P. D. Kaplan, H. A. Stone and D. A. Weitz, Science, 2005, 308, 537–541 CrossRef CAS PubMed.
- S. S. Lee, B. Kim, S. K. Kim, J. C. Won, Y. H. Kim and S.-H. Kim, Adv. Mater., 2015, 27, 627–633 CrossRef CAS PubMed.
- D. Lee and D. A. Weitz, Adv. Mater., 2008, 20, 3498–3503 CrossRef CAS.
- C. S. Ho, J. W. Kim and D. A. Weitz, J. Am. Chem. Soc., 2008, 130, 9543–9549 CrossRef PubMed.
- S.-H. Kim, J. W. Kim, D. H. Kim, S. H. Han and D. A. Weitz, Microfluid. Nanofluid., 2013, 14, 509–514 CrossRef CAS.
- W. Wang, M.-J. Zhang, R. Xie, X.-J. Ju, C. Yang, C.-L. Mou, D. A. Weitz and L.-Y. Chu, Angew. Chem., Int. Ed., 2013, 52, 8084–8087 CrossRef CAS PubMed.
- A. H. Mohammadi and D. Richon, Ind. Eng. Chem. Res., 2009, 48, 9045–9048 CrossRef CAS.
- S. S. Datta, S.-H. Kim, J. Paulose, A. Abbaspourrad, D. R. Nelson and D. A. Weitz, Phys. Rev. Lett., 2012, 109, 134302 CrossRef PubMed.
- S.-H. Kim, T. Y. Lee and S. S. Lee, Small, 2014, 10, 1155–1162 CrossRef CAS PubMed.
- R. McMullan and G. A. Jeffrey, J. Chem. Phys., 1959, 31, 1231–1234 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: SEM and optical microscope images of microcapsules fractured during hydrate formation and Movie S1 showing generation of double-emulsion drops. See DOI: 10.1039/c6ra19786h |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |