Cascade reaction for the construction of CF3 containing tetrasubstituted furan ring†
Abstract
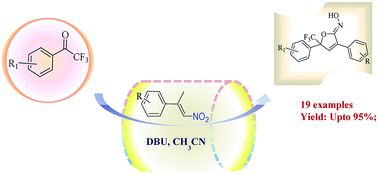
Activated olefins are well known nucleophiles, but proton abstraction from the substituted α-methyl group in nitroolefin is rare. We report the first DBU-catalysed one-pot reaction of TFMK and activated nitroolefin followed by intramolecular cyclization reaction, to construct stereogenic center containing a furan core with CF3 in excellent yields (up to >95%).


 Please wait while we load your content...
Please wait while we load your content...