Smart metallopoly(l-glutamic acid) polymers: reversible helix-to-coil transition at neutral pH†
Abstract
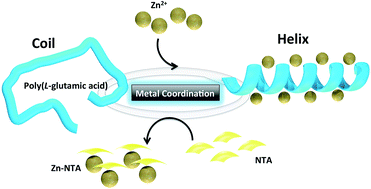
Among the smart polymers, smart polypeptides have a unique polymeric scaffold made of amino acids whose structuring can be controlled by an external stimuli. Herein we present how coordination chemistry to Zn species can be reversibly used to control the helix-to-coil transition of synthetic poly(L-glutamic acid) (PGA) polymers at a neutral pH of 7 in aqueous solutions.

 Please wait while we load your content...
Please wait while we load your content...