PW9V3/rGO/SPEEK hybrid material: an excellent proton conductor
Huaxue Caia,
Xuefei Wua,
Qingyin Wu*a,
Fahe Caoa and
Wenfu Yanb
aDepartment of Chemistry, Zhejiang University, Hangzhou 310027, China. E-mail: qywu@zju.edu.cn
bState Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P. R. China
First published on 31st August 2016
Abstract
To improve the proton conductive performance of heteropoly acids, sulfonated polyether ether ketone (SPEEK) and reduced graphene oxide (rGO) were introduced into a tungstovanadophosphoric acid (H6PW9V3O40, abbreviated as PW9V3) to prepare a hybrid film material. The results indicate that the Keggin framework of the PW9V3O406− anion still remains in the hybrid material and confirm the homogeneous dispersion of PW9V3 on the surface of graphene sheets, which results in a better stability of PW9V3. The obtained PW9V3/rGO/SPEEK hybrid material exhibits appreciable proton conductivity of 6.2 × 10−2 S cm−1 at 17 °C and 70% relative humidity because the introduction of rGO and SPEEK into PW9V3 can help to form more hydrogen bonds in the HPA-based material. The conductivity of the PW9V3/rGO/SPEEK hybrid material enhances with the increase of temperature, and it shows Arrhenius behavior with the activation energy value of 18.7 kJ mol−1, indicating that the proton conduction in this film occurs by a mixing mechanism. It is an alternative hybrid film material which may be applied in the field of proton exchange fuel cells.
1. Introduction
Heteropoly acids (HPAs) are an important class of nanosized polynuclear clusters composed of transition metals and oxygen atoms.1,2 HPAs are a versatile candidate as building blocks for the construction of hybrid materials due to their multiple charge transfer reactions and unique structural, electronic, and optical properties.3,4 Especially, owing to their high proton conductivity and excellent proton transfer/storage abilities, HPAs have become one of the best solid inorganic electrolytes for the development of fuel cells and supercapacitors.5,6 Despite these attractive features, the drawbacks of HPAs, for instance, high solubility in aqueous solutions, diffusional problems and continuous leakage, will result in the decrease of HPAs-based electrochemical devices, so thus, a pathway to address this problem is to construct HPAs-based hybrid materials.7,8Graphene has inspired great enthusiasm due to its excellent physical and chemical properties.9 For the large scale production of graphene-based materials, a widely adopted strategy is to use graphene oxide (GO) as a low cost precursor and convert it to reduced GO (rGO).10 Owing its exceptionally high specific surface area, rGO could fix HPAs even at very high loading by the electron transfer and electrostatic interaction between HPAs and the residual oxygen-containing groups of rGO;11 what's more, residual hydrophilic sites of rGO, such as –O–, –OH and –COOH functional groups, could contribute to the proton conductivity by forming hydrogen-bonds.12–15
Hence, based on HPAs with excellent conductivity, we chose SPEEK, which has been widely used as electrolyte film material in the field of fuel cell due to its good mechanical strength and high chemical stability,16,17 as the polymer matrix to prepare the PW9V3/rGO/SPEEK hybrid film material. The conductive properties of this film have been investigated.
2. Experimental
2.1. Instruments and reagents
Fourier-transform infrared spectroscopy (FTIR) was recorded on a NICOLET NEXUS470 FT/IR spectrometer using KBr as pellet, and the resolution is 4 cm−1. X-ray powder diffraction (XRD) was carried out on a BRUKER D8 ADVANCE X-ray diffractometer in the range of 2θ = 3–40° at the rate of 0.02° s−1. Morphology was observed by a Hitachi S-4800 (Japan) scanning electron microscope (SEM) and HF-3300 (Hitachi) transmission electron microscopy (TEM). Cyclic voltammetry (CV) was performed with a CHI660E electrochemical workstation in a conventional three-electrode electrochemical cell. Conductivity measurement was taken by a four-point-probe method using AC impedance spectroscopy over a frequency range of 100 mHz to 100 kHz, 10 mV AC perturbation. A sheet of film (4.5 cm × 1.5 cm) was placed on the test cell. Polyether ether ketone (PEEK) was obtained from College of Chemistry at Jilin University. Graphene oxide (GO) was synthesized by a new scalable and effective method.18 PW9V3 and SPEEK were synthesized by a modified method according to our literature procedures.19,20 The procedure from PEEK to SPEEK is shown in Fig. 1. The sulfonation degree (DS) was determined by an acid–base titration method. It is found that the DS is about 75%. All reagents are analysis grade.2.2. Preparation of PW9V3/rGO/SPEEK hybrid film material
0.35 g of PW9V3 powder was reduced by hydrazine hydrate to obtain the reduced PW9V3, which is often called ‘heteropoly blue’ (HB), and then dried at 55 °C. 10 mg of GO was dissolved in 30 mL deionized water, added the above reduced PW9V3 to GO solution and stirred for about 2 h. The solution turned black because the consecutive electron has transferred from HB to GO to produce the rGO. Afterwards, the solution was dried at 60 °C to get the PW9V3/rGO solid composite. 0.14 g of SPEEK was first dissolved in dimethylformamide (DMF) and added the above obtained PW9V3/rGO composite to the solution. The resulting mixture was mixed with the assistance of sonication and stirring for 4 h to form a suspension. After evaporation of most of the solvent, the mixture was cast onto a glass plate using a casting knife. Then the cast material was dried at room temperature for 48 h. The thickness of the obtained PW9V3/rGO/SPEEK hybrid film is 107 μm, and the film is flexible, black and homogeneous. The weight ratio of the film material is about 28% (SPEEK), 70% (PW9W3) and 2% (graphene). The procedure is depicted in Scheme 1.3. Results and discussion
Infrared spectrum is fairly useful for studies on properties of materials. Fig. 2 shows the IR spectra of pure GO nanosheets, PW9V3, PW9V3/rGO and PW9V3/rGO/SPEEK hybrid material. The spectrum of the GO shows the presence of O–H (νO–H at 3420 cm−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (νC
O (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1720 cm−1 from carboxyl group) and C–O (νC–O at 1060 cm−1 in alkoxy groups, at 1230 cm−1 in epoxy groups). While in PW9V3/rGO composite, the peak intensities of these oxygen-containing groups were decreased as a consequence of the deoxygenation process,21 indicating that GO, to some extent, has been reduced by heteropoly blue. The characteristic peaks of pure PW9V3 appear at 1070 cm−1, 979 cm−1, 881 cm−1 and 789 cm−1, which are assigned to νas(P–Oa), νas(M–Od), νas(M–Ob–M), and νas(M–Oc–M) of heteropolyanion PW9V3O406−. There are four similar peaks observed in the spectrum of SPEEK/PW9V3/rGO, they are 1079 cm−1, 962 cm−1, 901 cm−1 and 795 cm−1, respectively. It suggests that the Keggin framework of PW9V3O406− still maintain in the hybrid materials. What is worth notified is the peaks of νas(M–Od) in PW9V3/rGO/SPEEK have a red shift, from 979 to 962 cm−1. The reason is that the M–Od stretching is a proportional function of the anion–anion interaction. The addition of other material into PW9V3 would undoubtedly weaken the anion–anion interaction. Besides, the peaks observed at 1232 cm−1, 1019 cm−1 and 709 cm−1 are assigned to the stretching vibration of sulfonic acid groups (–SO3H) of SPEEK.22
O at 1720 cm−1 from carboxyl group) and C–O (νC–O at 1060 cm−1 in alkoxy groups, at 1230 cm−1 in epoxy groups). While in PW9V3/rGO composite, the peak intensities of these oxygen-containing groups were decreased as a consequence of the deoxygenation process,21 indicating that GO, to some extent, has been reduced by heteropoly blue. The characteristic peaks of pure PW9V3 appear at 1070 cm−1, 979 cm−1, 881 cm−1 and 789 cm−1, which are assigned to νas(P–Oa), νas(M–Od), νas(M–Ob–M), and νas(M–Oc–M) of heteropolyanion PW9V3O406−. There are four similar peaks observed in the spectrum of SPEEK/PW9V3/rGO, they are 1079 cm−1, 962 cm−1, 901 cm−1 and 795 cm−1, respectively. It suggests that the Keggin framework of PW9V3O406− still maintain in the hybrid materials. What is worth notified is the peaks of νas(M–Od) in PW9V3/rGO/SPEEK have a red shift, from 979 to 962 cm−1. The reason is that the M–Od stretching is a proportional function of the anion–anion interaction. The addition of other material into PW9V3 would undoubtedly weaken the anion–anion interaction. Besides, the peaks observed at 1232 cm−1, 1019 cm−1 and 709 cm−1 are assigned to the stretching vibration of sulfonic acid groups (–SO3H) of SPEEK.22
To test the electrochemical stability of PW9V3/rGO hybrid material, we have assembled the modified glassy carbon electrode (GCE) by depositing 5 μL of PW9V3/rGO dispersion (0.5 mg mL−1) on the surface of GCE, and casting 5 μL chitosan dispersion (0.01 g mL−1) on the surface of GCE to fix PW9V3/rGO. The result shows that the decay of the first reduction peak current was found to be only 4% after 100 circles (as shown in Fig. 3), and for comparison, the pure PW9V3 modified electrode is unstable when applied during the electrochemical study because of the high solubility in water of PW9V3. These observations indicate PW9V3 has been immobilized because of the strong interaction between PW9V3 and graphene. So graphene could act as a stabilizer to prevent PW9V3 from leaching out. It is more favorable when PW9V3/rGO rather pure PW9V3 applied in SPEEK/PW9V3/rGO hybrid material.23
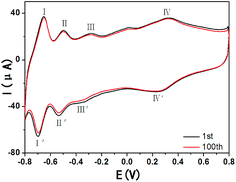 | ||
| Fig. 3 CV stability test of PW9V3/rGO for 100 cycles at the scan rate of 90 mV s−1 in 0.025 M H2SO4 electrolyte. | ||
TEM image was used to characterize the morphology of the as-obtained PW9V3/rGO/SPEEK hybrid material. Fig. 4a illustrated the wrinkled and flake-like shape at high magnification, which is the feature structure of graphene nanosheets, indicating that graphene disperses well in the hybrid matrix. In Fig. 4b, the presence of the inorganic PW9V3 clusters in hybrid film is clearly detected as small dots. This homogeneous distribution will be beneficial to the proton mobility as it minimizes the distance between particles.
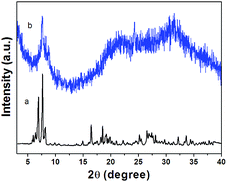
Fig. 5 represents the X-ray powder diffraction patterns of PW9V3 and PW9V3/rGO/SPEEK. Compared with PW9V3, the most intense characteristic peaks still identified in PW9V3/rGO/SPEEK composites at the range of 2θ = 7–11°. It indicates the existence of Keggin anion in the hybrid materials. This is consistent with the results of the IR. The broad diffraction peaks at 15–38° were observed for PW9V3/rGO/SPEEK, suggesting that the hybrid material is considered amorphous.
The proton conductivity of hybrid materials has been investigated by electrochemical impedance spectroscopy. Fig. 6 shows Nyquist plots of SPEEK/PW9V3/rGO at room ambient of 17 °C and 60% relative humidity. Inset is the equivalent circuit. The high-frequency semicircle of Nyquist plots is modeled by parallel of a resistor R with a constant phase element CPE1. R is the actual resistance of the film material, and the low-frequency inclined line of Nyquist plots represents the impedance of the film/electrodes interfaces and is modeled by CPE2. The proton conductivity (σ, S cm−1) of film material was calculated using the following equation: σ = L/(RS) (L is the distance between the electrode pairs, S is the cross-sectional area of the film and R is the resistance of the film). By calculation, conductivity of PW9V3/rGO/SPEEK is 6.2 × 10−2 S cm−1. For comparison, we have also got the conductivities of pure SPEEK film and PW9V3/SPEEK (70 wt% PW9V3) film, which are 1.1 × 10−4 S cm−1 and 3.76 × 10−2 S cm−1 at the ambient condition. So it is obvious PW9V3/rGO/SPEEK hybrid shows the highest conductivity. The –O–, –OH and –COOH functional groups of rGO have hydrophilic sites,24,25 and SPEEK is composed of a hydrophobic backbone and ionic domains filled with –SO3H in the hydrated state (see the structure of SPEEK in Fig. 1). So the introduction of rGO and SPEEK into PW9V3 can help to form more hydrogen bonds in the system to enhance the water retention in the HPA-based materials and accelerate the proton conduction.
 | ||
| Fig. 6 Electrochemical impedance spectrum of PW9V3/rGO/SPEEK film material at 17 °C and 70% relative humidity. | ||
To investigate the relationship between proton conductivity with temperature, we have measured the conductivity in the range of 17–70 °C. It is found that the proton conductivity of SPEEK/PW9V3/rGO increases with higher temperature as the mobility of conducting species increase with higher temperature, which increases to 2.36 × 10−1 S cm−1 at 70 °C. As shown in Fig. 7, the relationship between proton conductivity and temperature is consistent with Arrhenius equation: σ = σ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(Ea/κT). In this formula, Ea is the activation energy of proton conductivity, σ0 is the pre-exponential factor and κ is the Boltzmann constant. The activation energy of PW9V3/rGO/SPEEK calculated from the slope is 18.7 kJ mol−1. It is lower than pure PW9V3, whose is 25.68 kJ mol−1.19 It indicates the influence of temperature on the conductivity decreases when pure PW9V3 hybrids with rGO and polymer.
exp(Ea/κT). In this formula, Ea is the activation energy of proton conductivity, σ0 is the pre-exponential factor and κ is the Boltzmann constant. The activation energy of PW9V3/rGO/SPEEK calculated from the slope is 18.7 kJ mol−1. It is lower than pure PW9V3, whose is 25.68 kJ mol−1.19 It indicates the influence of temperature on the conductivity decreases when pure PW9V3 hybrids with rGO and polymer.
So far, there are two major recognized proton conduction mechanisms: vehicle mechanism and Grotthuss mechanism.26 In vehicle mechanism, protons interact with water molecules, which transfer in the form of hydrated hydrogen ions, such as H3O+, H5O2+ and H9O4+ species, similar to molecular diffusion, it differs from Grotthuss mechanism, in which a large amount of water can assist proton hopping from one proton carrier to a neighboring one down a chain of hydrogen-bonded network. Therefore, water plays a fairly important role in the process of proton mobility.27 Generally, we distinguish them by the value of activation energy, because the activation energy of vehicle mechanism is often higher than 20 kJ mol−1, while the activation energy of Grotthuss mechanism is usually less than 15 kJ mol−1. The activation energy of this hybrid material is 18.7 kJ mol−1, indicating the proton conduction of PW9V3/rGO/SPEEK film material is a share of Grotthuss mechanism and vehicle mechanism. Just as shown in Fig. 8, the blue arrow is the track of proton-hopping in the network structure, and the red arrow represents the pathway of proton movement with the assistance of water. It is the mixing mechanism that accelerates the proton conduction in this hybrid film.
4. Conclusions
In this work, a proton-conducting hybrid film material PW9V3/rGO/SPEEK was formed. The results indicate that the Keggin framework of PW9V3O406− anion still remain in the hybrid film and confirm the homogeneous dispersion of PW9V3 on the surface of graphene sheet. This hybrid film material shows a superior proton conductivity of 6.2 × 10−2 S cm−1 at ambient condition and the activation energy of proton conduction are 18.7 kJ mol−1, indicating it is the mixing mechanism that accelerates the proton conduction in this hybrid film. So it is a high proton conductor. This work lays a solid foundation in the field of HPAs-based materials, especially in the special fields of proton exchange membrane fuel cells and supercapacitors.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21071124, 21173189), the Zhejiang Provincial Natural Science Foundation of China (LY14B030005) and the Foundation of State Key Laboratory of Inorganic Synthesis, Preparative Chemistry of Jilin University (2016-03).Notes and references
- Z. X. Sun, Y. Z. Zhang, N. Li, L. Xu and T. Q. Wang, J. Mater. Chem. C, 2015, 3, 6153–6157 RSC.
- J. P. Tessonnier, S. Goubert-Renaudin, S. Alia, Y. S. Ya and M. A. Barteau, Langmuir, 2013, 29, 393–402 CrossRef CAS PubMed.
- A. Rubinstein, P. Jiménez-Lozanao, J. J. Carbó, J. M. Poblet and R. Neumann, J. Am. Chem. Soc., 2014, 136, 10941–10948 CrossRef CAS PubMed.
- X. F. Wu, X. Tong, Q. Y. Wu, H. Ding and W. F. Yan, J. Mater. Chem. A, 2014, 2, 5780–5784 CAS.
- S. Y. Oh, T. Yoshida, G. Kawamura, H. Muto, M. Sakai and A. Matsuda, J. Power Sources, 2010, 195, 5822–5828 CrossRef CAS.
- M. H. Yang, B. G. Choi, S. C. Jung, Y. K. Han, Y. S. Huh and S. B. Lee, Adv. Funct. Mater., 2014, 24, 7301–7309 CrossRef CAS.
- Y. Kim, K. Ketpang, S. Jaritphun, J. S. Park and S. Shanmugam, J. Mater. Chem. A, 2015, 3, 8148–8155 CAS.
- M. H. Yang, D. S. Kim, T. J. Lee, S. J. Lee, K. G. Lee and B. G. Choi, J. Colloid Interface Sci., 2016, 468, 51–56 CrossRef CAS PubMed.
- H. L. Li, S. P. Pang, S. Wu, X. L. Feng, K. Müullen and C. Bubeck, J. Am. Chem. Soc., 2011, 133, 9423–9429 CrossRef CAS PubMed.
- S. Wang, H. L. Li, L. Y. Zhang, B. Li, X. Cao, G. H. Zhang, S. L. Zhang and L. X. Wu, Chem. Commun., 2014, 50, 9700–9703 RSC.
- W. H. Guo, X. H. Cao, Y. H. Liu, X. L. Tong and X. S. Qu, J. Electrochem. Soc., 2014, 161, 248–255 CrossRef.
- Y. W. Liu, S. M. Liu, X. Y. Lai, J. Miao, D. F. He, N. Li, F. Luo, Z. Shi and S. X. Liu, Adv. Funct. Mater., 2015, 25, 4480–4485 CrossRef CAS.
- Y. H. Lee, K. H. Chang and C. C. Hu, J. Power Sources, 2013, 227, 300–308 CrossRef CAS.
- Y. Y. Shao, J. Wang, M. Engelhard, C. Wang and Y. H. Lin, J. Mater. Chem., 2010, 20, 743–748 RSC.
- X. F. Lei, Y. H. Chen, M. T. Qiao, L. D. Tian and Q. Y. Zhang, J. Mater. Chem. C, 2016, 4, 2134–2146 RSC.
- N. Zhang, G. Zhang, D. Xu, C. J. Zhao, W. J. Ma and H. T. Li, Int. J. Hydrogen Energy, 2011, 36, 11025–11033 CrossRef CAS.
- T. Yang, J. Membr. Sci., 2009, 342, 221–226 CrossRef CAS.
- L. Peng, Z. Xu, Z. Liu, Y. Y. Wei, H. Y. Sun, Z. Li, X. L. Zhao and C. Gao, Nat. Commun., 2015, 6, 5716, DOI:10.1038/ncomms6716.
- (a) X. Tong, N. Q. Tian, W. Wu, W. M. Zhu, Q. Y. Wu, F. H. Cao, W. F. Yan and A. B. Yaroslavtsev, J. Phys. Chem. C, 2013, 117, 3258–3263 CrossRef CAS; (b) Q. Y. Wu, X. Y. Qian and S. M. Zhou, J. Xuzhou Inst. Technol., Nat. Sci. Ed., 2012, 27, 1–4 CAS.
- (a) N. Q. Tian, X. F. Wu, B. H. Yang, Q. Y. Wu, F. H. Cao, W. F. Yan and A. B. Yaroslavtsev, J. Appl. Polym. Sci., 2015, 132, 42204 Search PubMed; (b) Q. Y. Wu, X. Tong and X. F. Wu, J. Xuzhou Inst. Technol., Nat. Sci. Ed., 2011, 26, 1–8 Search PubMed.
- M. H. Yang, B. G. Choi, H. S. Park, W. H. Hong, S. Y. Lee and T. J. Park, Electroanalysis, 2010, 22, 1223–1228 CrossRef CAS.
- R. Kumar, M. Mamlouk and K. Scott, RSC Adv., 2014, 4, 617–623 RSC.
- Y. Kim, K. Ketpang, S. Jaritphun, J. S. Park and S. Shanmugam, J. Mater. Chem. A, 2015, 3, 8148–8155 CAS.
- A. Khan, R. Asmatulu and G. Hwang, ECS Trans., 2015, 69, 569–577 CrossRef.
- J. L. Zhang, H. J. Yang, G. X. Shen, P. Cheng, J. Y. Zhang and S. W. Guo, Chem. Commun., 2010, 46, 1112–1114 RSC.
- K. Cheückiewicz, G. Zúukowska and W. Wieczorek, Chem. Mater., 2001, 13, 379–384 CrossRef.
- A. Eguizábal, J. Lemus, M. Urbiztondo, O. Garrido, J. Soler, J. A. Blazquez and M. P. Pina, J. Power Sources, 2011, 196, 8994–9007 CrossRef.
| This journal is © The Royal Society of Chemistry 2016 |