A review on Mn4+ activators in solids for warm white light-emitting diodes
Abstract
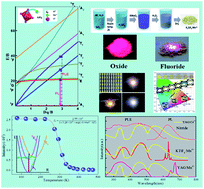
Currently, the major commercial white light-emitting diodes consist of a blue-emitting chip and Y3Al5O12:Ce3+ yellow phosphor. However, the shortage of a red emitting component in the constructed device makes it difficult to realize warm white light with a high color-rendering index and low correlated color temperature. In this mini-review article, we provide an overview of recent progresses in developing Mn4+ doped red phosphors for promising applications in warm white light-emitting diodes. Firstly, the spectroscopic properties of Mn4+ in solids, including electronic and vibronic energy-level structures, crystal-field parameters as well as thermal stability, were briefly discussed. And then the related physical and chemical synthesis strategies were introduced in detail. Afterwards, Mn4+ doped phosphors, such as oxides, fluorides as well as glass ceramic composites, and their impact on improving the photoelectric performance of white light-emitting diodes were summarized. Finally, several challenges and perspectives for exploring novel and high-performance Mn4+ doped red phosphors will be presented.

 Please wait while we load your content...
Please wait while we load your content...