Highly-ordered microporous carbon nanospheres: a promising anode for high-performance sodium-ion batteries
Qiliang Weia,
Yanqing Fuba,
Gaixia Zhanga,
Youling Wanga,
Xianyou Wang*b,
Mohamed Mohamedia and
Shuhui Sun*a
aInstitut National de la Recherche Scientifique-Énergie Matériaux et Télécommunications, Varennes, QC J3X 1S2, Canada. E-mail: shuhui@emt.inrs.ca
bKey Laboratory of Environmentally Friendly Chemistry and Applications of Ministry of Education, Hunan Province Key Laboratory for Electrochemical Energy Storage and Conversion, School of Chemistry, Xiangtan University, Xiangtan 411105, Hunan, China. E-mail: wxianyou@yahoo.com
First published on 31st August 2016
Abstract
Highly-ordered microporous carbon (MPC) nanospheres were prepared by a simple hydrothermal route based on a soft template. The interlayer spacing of the MPC was well tuned by simply adjusting the heat-treatment temperatures. The as-obtained carbon spheres treated at 700 °C (MPC-700) combine the features required for high-performance sodium-ion battery (SIB) electrode materials, such as a large interlayer spacing (∼0.457 nm), high surface area, structural stability and plenty of micropores for Na insertion, which synergistically contribute to their impressive electrochemical properties. When applied as an anode for SIBs, the MPC-700 electrode exhibits a high reversible capacity, good cycling stability, and an excellent high-rate performance (∼160 mA h g−1 after 500 cycles at 1000 mA g−1), making it a promising candidate for SIB anodes.
1. Introduction
Sodium ion batteries (SIBs) are drawing increasing attention as a promising alternative to lithium ion batteries (LIBs) owing to their evident advantages such as lower production cost, natural Na abundance and environmental benignity.1–6 Nevertheless, the development of SIBs is hampered by the lack of high-performance electrode materials. As a result, great efforts have been made to explore suitable electrode materials for SIBs. Recently, exciting progress on cathode materials has been achieved by some groups.7–15 However, the discovery of high performance anode materials is still challenging. Among various candidates investigated, carbon-based materials such as hard carbon,6,16–19 carbon black,20 carbon microspheres,21–23 hollow carbon nanowires,24 carbon nanofibers,25,26 carbon nanosheets,27,28 carbon nanotubes,29 and porous carbon,30–34 have been investigated as anode candidates for SIBs. It is well accepted that the large interlayer distance and disordered structure of hard carbon could facilitate Na+ insertion/extraction.24,35 For example, the initial capacities of 300 mA h g−1 and 285 mA h g−1 for hard carbon were reported by Stevens et al., and Alcántara et al., respectively, but only with limited cycles.17,21 Tang et al.36 reported that polystyrene-templated hollow carbon microspheres possess large interlayer spacing (∼0.401 nm), leading to excellent performance for SIBs, i.e. 160 mA h g−1 at 100 mA g−1 over 100 cycles. Later, the carbon nanofibers reported by Cao et al.24 and Luo et al.25 displayed excellent cycling stability, while the rate capability is not very competitive. Moreover, it has been reported that the nanopores resulted from the randomly scattered graphene nanodomains in porous carbon can effectively store Na+, facilitating fast kinetics and high capacity.17,34,37 For example, the mesoporous carbon synthesized based on silica hard template exhibited significantly improved rate performance, exceeding 100 mA h g−1 even at high rates,34 however, the charge/discharge was reported with only 40 cycles. What's more, the hard-templating method is well known for its tedious steps. This encourages us to explore simpler method to fabricate porous structures and to systematically study the effect of pore size and investigate whether further increasing the interlayer spacing in the porous structure will be more effective for Na-ion storage, and therefore benefit for a higher reversible capacity and better rate performance.Herein, we report the use of highly ordered microporous carbon (MPC) spheres, synthesized based on soft-template, as a high-performance anode material for SIB. The highly-ordered MPC spheres are rich in micropores and possess large and tunable interlayer spacing, which provides a harmonious electrochemical environment that guarantees smooth Na-ion transfer and high charge storage capability. The results show that the MPCs after treatment of 700 °C exhibit the best electrochemical performance, making them promising candidates for SIB anodes.
2. Experimental
2.1. Synthesis of highly-ordered MPC spheres
All the chemicals are analytical grade and used as received. The samples were prepared by a low-concentration hydrothermal route.38,39 Firstly, a tri-block copolymer Pluronic F127 (0.96 g, Mw ∼ 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, Sigma-Aldrich Cor.), acting as soft template, was dissolved in 15 mL DI water at room temperature by stirring for 30 min, then phenol (0.6 g), formaldehyde solution (2.1 mL), 1,10-phenanthroline (100 mg), and 0.1 M NaOH aqueous solution (15 mL) were mixed and stirred at 70 °C for another 30 min to obtain a low-molecular-weight phenolic resols. After that, the dissolved F127 solution was dropped to the phenolic resols slowly, and 2 h later, 50 mL of water was added and further stirred at 70 °C for 12–14 h. Afterwards, 15 mL of the as-prepared monomicelle solution and 22 mL of H2O was transferred into an autoclave (50 mL volume) for hydrothermal treatment at 130 °C for 20 h. In the end, the products were collected by centrifugation and washed with distilled water for several times, and then dried in an oven at 60 °C. The samples were first carbonized at 450 °C for 2 h and then further carbonized at different temperatures (550 °C, 700 °C, 850 °C and 1050 °C) for additional 2 h, thus, the corresponding samples were named as MPC-550, MPC-700, MPC-850 and MPC-1050, respectively.
500, Sigma-Aldrich Cor.), acting as soft template, was dissolved in 15 mL DI water at room temperature by stirring for 30 min, then phenol (0.6 g), formaldehyde solution (2.1 mL), 1,10-phenanthroline (100 mg), and 0.1 M NaOH aqueous solution (15 mL) were mixed and stirred at 70 °C for another 30 min to obtain a low-molecular-weight phenolic resols. After that, the dissolved F127 solution was dropped to the phenolic resols slowly, and 2 h later, 50 mL of water was added and further stirred at 70 °C for 12–14 h. Afterwards, 15 mL of the as-prepared monomicelle solution and 22 mL of H2O was transferred into an autoclave (50 mL volume) for hydrothermal treatment at 130 °C for 20 h. In the end, the products were collected by centrifugation and washed with distilled water for several times, and then dried in an oven at 60 °C. The samples were first carbonized at 450 °C for 2 h and then further carbonized at different temperatures (550 °C, 700 °C, 850 °C and 1050 °C) for additional 2 h, thus, the corresponding samples were named as MPC-550, MPC-700, MPC-850 and MPC-1050, respectively.
2.2. Physical characterizations
The crystal structure of the as-prepared samples was characterized by X-ray diffraction (XRD, Bruker D8 Advanced Diffractometer, Cu Kα radiation). The morphological structures were observed by transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) (JEOL JEM-2100F, operated at 200 kV). The specific surface area of the as-prepared sample was determined by N2 adsorption/desorption isotherm at 77 K (JW-BK112), and the pore size distribution was calculated by analyzing the adsorption branch of the N2 isotherms using the quenched solid-state density functional theory (QSDFT) method. Raman spectroscopy (Renishaw Imaging Microscope Wire™) was performed using the 785 nm laser radiation with a circular polarization. The laser beam was focused onto the sample to a spot size of 1 μm.2.3. Electrochemical measurements
The button-type cells were assembled in an argon-filled glove box, where water and oxygen concentration were kept less than 5 ppm. The working electrodes were fabricated by mixing 70 wt% of active materials, 20 wt% of acetylene black and 10 wt% of polymer binder (polyvinylidene fluoride, PVDF), which were then pasted on copper foil followed by drying under vacuum at 110 °C for 10 h. The mass loading of active material on each disk is about 1.3 mg cm−2. Metal sodium was used as negative electrode; the electrolyte was 1 M NaClO4 in propylene carbonate (PC) solution; the separator was glass microfiber filters (Whatman, GF/D). The galvanostatic charge–discharge measurements were performed using a Battery Testing System (Neware BTS-4008) at different current densities with a cut-off voltage window of 0–3.0 V. Cyclic Voltammetry (CV) tests were carried out on Autolab electrochemical workstation at a scan rate of 0.1 mV s−1 with the potential interval 0–3.0 V. Electrochemical impedance spectroscopy (EIS) was employed to measure the assembled coin cell on Autolab electrochemical workstation by applying an AC amplitude of 5 mV over the frequency range from 105 to 0.01 Hz at an open circuit voltage (OCV: about 2.0 V vs. Na+/Na). All the electrochemical measurements were performed at room temperature.3. Results and discussion
The morphologies of the as-synthesized materials were investigated by TEM. As shown in Fig. 1a, the product MPC-700 is composed of homogeneous spheres with diameter in the range of 100–120 nm with highly ordered pores. The HRTEM image (Fig. 1b) further demonstrates the rough surface and highly porous structure of the carbon sphere, which is favourable for Na+ diffusion from various orientations and sufficient contact between active materials and electrolyte.40 Fig. 1c is the XRD patterns of MPC samples after different temperature treatment. For MPC-550, there is almost no obvious peaks appear, indicating its amorphous and disorder state which is probably caused by the low temperature treatment. From MPC-700 to MPC-1050, the (002) diffraction peak gradually shifts to higher angle, indicating that the spacing shrinkage between adjacent sheets occurs at higher temperature. According to Bragg equation, the interlayer spacing (d002) was calculated to be 0.457 nm for MPC-700, (versus 0.422 nm for MPC-850 and 0.403 nm for MPC-1050). More importantly, the interlayer spacing distance of MPC-700 in this work is larger than most reported carbon materials for SIBs.24–29,33–41 The larger free space are expected to be favourable for the reversible storage of sodium considering the larger diameter of Na+ compared to that of Li+.36 The Raman spectrum of MPC-700 shows broad D and G bands at around 1313 and 1593 cm−1, respectively (Fig. 1d). Notably, the MPC-700 sample exhibits very high peak intensity ratio of the D to G band (ID/IG = 1.51), which is probably caused by the edge defects of the micropore structure. It has been reported that the edge defects could enhance the activity of the electrodes,42–45 thus should be beneficial to the electrochemical performance. | ||
| Fig. 1 (a) TEM image and (b) HR-TEM image of MPC-700 spheres, (c) XRD pattern of MPC spheres at different treating temperature, (d) Raman spectrum of MPC-700. | ||
The pore-size distribution and porous structure were analysed by nitrogen adsorption–desorption isotherms. Fig. 2a presents the Type I isotherm with a sharp uptake at low pressure and a smooth plateau at middle-to-high pressure, which is characteristic of microporous materials.46 The specific surface area of MPC samples decreased as the increase of heat-treatment temperature (from 642.5 m2 g−1 for MPC-550, 580.1 m2 g−1 for MPC-700, 551.7 m2 g−1 for MPC-850, to 485.2 m2 g−1 for MPC-1050) (Fig. 2a), this is probably caused by the shrinkage between adjacent sheets at higher temperatures as indicated in XRD (Fig. 1c). As revealed by the QSDFT pore size distribution (Fig. 2b), all samples possess plenty of micropores with size at around 0.6 nm and a small number of mesopores ranging between 2.0–3.0 nm. It has been reported that micropores with narrow pore-size distribution are beneficial for the reversible storage of Na+.30
 | ||
| Fig. 2 (a) N2 sorption isotherm of MPC samples, (b) pore-size distribution curves of MPC samples calculated by QSDFT. | ||
CV and galvanostatic charge/discharge cycling were performed to investigate the Na+ insertion/extraction properties (Fig. 3). As shown in Fig. 3a, in the first CV cycle, apparent cathodic peaks are observed at 0.8 V, 0.46 V and near 0 V for MPC-700. The peaks at around 0.8 V and 0.46 V come from the decomposition of electrolyte and formation of a solid electrolyte interphase (SEI).45 The clear cathodic peak near 0 V is ascribed to Na+ insertion into porous carbon. For the anodic processes, the main Na+ extraction peak locates at around 0.08 V and the sodium removal occurs over a broad potential range (0.2 to 0.8 V), which is similarly to the previously reported work.28,36,37 Moreover, it can be seen that the CV curves of the 3rd and 4th cycles almost overlap each other, indicating the excellent reversibility of Na+ insertion/extraction in MPC-700.
As shown in Fig. 3b, the first discharge/charge cycle of MPC-700 delivers specific discharge/charge capacity of 465 and 237 mA h g−1, respectively (with an initial coulombic efficiency of 51.0%), at 100 mA g−1. Similar to that reported in LIBs, the large irreversible capacity in the first cycle is an unavoidable phenomenon in porous carbonaceous electrodes. In addition to the electrolyte decomposition at the electrode/electrolyte interface and the formation of SEI films, the deep-seated micropores may also lead to irreversible sodium insertion/extraction in MPCs.37,47 This can be also verified with the CV of MPC-700 at the first scanning cycle. From Fig. 3b and c, it can be seen that MPC-700 shows excellent capacity retention with a high reversible capacity of ∼180 mA h g−1 after 100 cycles at 100 mA g−1, the coulombic efficiency approaches to ∼100% after several cycles. For comparison, after 100 cycles, MPC-550, MPC-850 and MPC-1050 maintain reversible capacities of 137, 162 and 98 mA h g−1, respectively, showing that 700 °C is an optimal heat-treatment temperature for the good performance of SIBs. It should be noted that the capacity of MPC-550 decreases faster than other three samples, this might be caused that with the highest surface area, the risk of secondary reactions involving electrolyte decomposition between electrode and electrolyte raises, this could cause a high level of irreversibility and poor cycle life.48 While, the MPC-1050 shows much lower capacity which is probably ascribed to the smaller surface area and interlayer spacing. MPC-700 shows the best electrochemical performance among the four samples which can be attributed to the following: (i) its larger interlayer spacing of d002 (0.457 nm) favorites the smooth reversible storage of sodium; (ii) the suitable porous structure with crystallographic defects (as shown in Fig. 1b) serve as reservoirs for storage of sodium; (iii) an optimal balance between high surface area (for sufficient interface contact between the electrode and electrolyte) and good electrical conductivity (for electron transport).
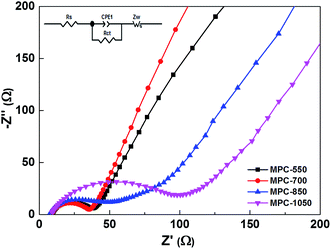
Rate capability is another important index to evaluate the performance of SIBs. As shown in Fig. 3d, the reversible capacities of MPC-700 sample are retained at 230, 210, 190, 165, 132, 125 mA h g−1 at current densities of 100, 200, 500, 1000, 2000, 3000 mA g−1, respectively. Moreover, when the current density goes back to 100 mA g−1, the capacity recovers to 210 mA h g−1, which is higher than those of the other three samples at all different stages. It should be noted that for the MPC-550 sample, at the identical current densities, the capacities are 200, 180, 145, 119, 90 and 68 mA h g−1, respectively. At larger current densities, the capacity decreases dramatically, which can be ascribed to its poor conductivity due to the low treatment temperature. While, when the treating temperature goes up to 850 and 1050 °C, although the performance retains relatively well at higher current densities, the capacity is lower due to the smaller surface area and interlayer spacing. Therefore, by combining the multifactor functioning effects, it can be concluded that the 700 °C is the optimal annealing temperature of MPC for use in SIBs. Furthermore, long term cycling of the MPC-700 at 1000 mA g−1 exhibits a stable capacity of ∼160 mA h g−1 over 500 cycles (Fig. 3e), indicating that the large interlayer distance guarantees the structural stability during the repeated sodiation/desodiation processes. To better understand the higher performance of the microporous spheres for SIBs, the EIS measurements of MPC samples (Fig. 4) were carried out on cells comprising the samples as the working electrode vs. Na. Both of the Nyquist plots are similar to each other, composing of a broad depressed semicircle at high-medium frequency that is related to the ohmic resistance and charge transfer resistance (Rct), and an inclined straight line at low frequency that is associated with ionic diffusion impedance through the bulk of the active material. The Rct of MPC-700 is about 26 Ω which is smaller than those for the other three MPC samples, indicating MPC-700 has much lower activation energy for Na+ diffusion and undergoes a fast faradaic reaction, conforming the excellent electrochemical properties.
The excellent electrochemical performance of MPC-700 is attributed to the specific characteristics of its unique spherical structure with plentiful micropores and suitable surface area. Firstly, thanks to the spherical shape, MPCs tend to form thin film electrodes without a preferred orientation, which is beneficial to homogeneous current low distribution within the electrode.23 Secondly, the isotropic spherical structure is beneficial for high packing density and rate performance of the electrodes.49 Thirdly, the large interlayer spacing of the disordered structure facilitates sodium ion transport and storage. Finally, suitable surface area provides more active sites for the Na+ storage, meanwhile, it astricts secondary reactions involving electrolyte decomposition between electrode and electrolyte.
4. Conclusions
In summary, we employed a simple soft-template method to fabricate highly-ordered spherical MPCs. The interlayer spacing and surface area can be tuned by simply adjusting the annealing temperature, which has large effects to the electrochemical performances. When used as anodes for SIBs, the synergistic effect of larger interlayer spacing, suitable surface area, and the numerous micropores in MPC-700 facilitates Na-ion transport and storage, contributing to excellent cycling stability and rate capability. Further optimization of such a promising electrode material can be conducted by adjusting their size, the ratio of micropores/mesopores and hetero-element doping (such as N, B, P, S…), which are believed to be effective strategies to boost the developments of new carbon materials for SIBs.Acknowledgements
This work is financially supported by the Natural Sciences and Engineering Research Council of Canada (NSERC), Institut National de la Recherche Scientifique (INRS), and Centre Québécois sur les Materiaux Fonctionnels (CQMF). Q. Wei gratefully acknowledges the scholarship from China Scholarship Council (CSC) and Fonds de Recherche du Québec-Nature et Technologies (FRQNT).References
- J. M. Tarascon, Nat. Chem., 2010, 2, 510 CrossRef CAS PubMed
.
- B. Scrosati and J. Garche, J. Power Sources, 2010, 195, 2419–2430 CrossRef CAS
.
- N. Yabuuchi, K. Kubota, M. Dahbi and S. Komaba, Chem. Rev., 2014, 114, 11636–11682 CrossRef CAS PubMed
.
- C. Y. Yu, J. S. Park, H. G. Jung, K. Y. Chung, D. Aurbach, Y. K. Sun and S. T. Myung, Energy Environ. Sci., 2015, 8, 2019–2026 CAS
.
- D. Kundu, E. Talaie, V. Duffort and L. F. Nazar, Angew. Chem., Int. Ed., 2015, 54, 3431–3448 CrossRef CAS PubMed
.
- S. W. Kim, D. H. Seo, X. Ma, G. Ceder and K. Kang, Adv. Energy Mater., 2012, 2, 710–721 CrossRef CAS
.
- D. Kim, S. H. Kang, M. Slater, S. Rood, J. T. Vaughey, N. Karan, M. Balasubramanian and C. S. Johnson, Adv. Energy Mater., 2011, 1, 333–336 CrossRef CAS
.
- Y. Yamada, T. Doi, I. Tanaka, S. Okada and J. I. Yamaki, J. Power Sources, 2011, 196, 4837–4841 CrossRef CAS
.
- P. Barpanda, T. Ye, S. I. Nishimura, S. C. Chung, Y. Yamada, M. Okubo, H. Zhou and A. Yamada, Electrochem. Commun., 2012, 24, 116–119 CrossRef CAS
.
- J. Kang, S. Baek, V. Mathew, J. Gim, J. Song, H. Park, E. Chae, A. K. Rai and J. Kim, J. Mater. Chem., 2012, 22, 20857–20860 RSC
.
- D. Kim, E. Lee, M. Slater, W. Lu, S. Rood and C. S. Johnson, Electrochem. Commun., 2012, 18, 66–69 CrossRef CAS
.
- J. Billaud, G. Singh, A. R. Armstrong, E. Gonzalo, V. Roddatis, M. Armand, T. Rojo and P. G. Bruce, Energy Environ. Sci., 2014, 7, 1387–1391 CAS
.
- J. Billaud, R. J. Clément, A. R. Armstrong, J. Canales-Vázquez, P. Rozier, C. P. Grey and P. G. Bruce, J. Am. Chem. Soc., 2014, 136, 17243–17248 CrossRef CAS PubMed
.
- Y. You, X. L. Wu, Y. X. Yin and Y. G. Guo, Energy Environ. Sci., 2014, 7, 1643–1647 CAS
.
- J. Y. Hwang, S. M. Oh, S. T. Myung, K. Y. Chung, I. Belharouak and Y. K. Sun, Nat. Commun., 2015, 6, 6865 CrossRef CAS PubMed
.
- S. Komaba, W. Murata, T. Ishikawa, N. Yabuuchi, T. Ozeki, T. Nakayama, A. Ogata, K. Gotoh and K. Fujiwara, Adv. Funct. Mater., 2011, 21, 3859–3867 CrossRef CAS
.
- D. A. Stevens and J. R. Dahn, J. Electrochem. Soc., 2000, 147, 1271–1273 CrossRef CAS
.
- A. Ponrouch, A. R. Goñi and M. R. Palacín, Electrochem. Commun., 2013, 27, 85–88 CrossRef CAS
.
- C. Bommier, W. Luo, W. Y. Gao, A. Greaney, S. Ma and X. Ji, Carbon, 2014, 76, 165–174 CrossRef CAS
.
- R. Alcántara, J. M. Jiménez-Mateos, P. Lavela and J. L. Tirado, Electrochem. Commun., 2001, 3, 639–642 CrossRef
.
- R. Alcántara, P. Lavela, G. F. Ortiz and J. L. Tirado, Electrochem. Solid-State Lett., 2005, 8, A222–A225 CrossRef
.
- V. G. Pol, E. Lee, D. Zhou, F. Dogan, J. M. Calderon-Moreno and C. S. Johnson, Electrochim. Acta, 2014, 127, 61–67 CrossRef CAS
.
- T. Chen, L. Pan, T. Lu, C. Fu, D. H. C. Chua and Z. Sun, J. Mater. Chem. A, 2014, 2, 1263–1267 CAS
.
- Y. Cao, L. Xiao, M. L. Sushko, W. Wang, B. Schwenzer, J. Xiao, Z. Nie, L. V. Saraf, Z. Yang and J. Liu, Nano Lett., 2012, 12, 3783–3787 CrossRef CAS PubMed
.
- W. Luo, J. Schardt, C. Bommier, B. Wang, J. Razink, J. Simonsen and X. Ji, J. Mater. Chem. A, 2013, 1, 10662–10666 CAS
.
- W. Li, L. Zeng, Z. Yang, L. Gu, J. Wang, X. Liu, J. Cheng and Y. Yu, Nanoscale, 2014, 6, 693–698 RSC
.
- H. G. Wang, Z. Wu, F. L. Meng, D. L. Ma, X. L. Huang, L. M. Wang and X. B. Zhang, ChemSusChem, 2013, 6, 56–60 CrossRef PubMed
.
- F. Yang, Z. Zhang, K. Du, X. Zhao, W. Chen, Y. Lai and J. Li, Carbon, 2015, 91, 88–95 CrossRef CAS
.
- D. Li, L. Zhang, H. Chen, L. X. Ding, S. Wang and H. Wang, Chem. Commun., 2015, 51, 16045–16048 RSC
.
- Q. Qu, J. Yun, Z. Wan, H. Zheng, T. Gao, M. Shen, J. Shao and H. Zheng, RSC Adv., 2014, 4, 64692–64697 RSC
.
- Z. Wang, Y. Li and X. J. Lv, RSC Adv., 2014, 4, 62673–62677 RSC
.
- Y. Kado, Y. Soneda and N. Yoshizawa, ECS Electrochem. Lett., 2015, 4, A22–A23 CrossRef CAS
.
- Z. Guan, H. Liu, B. Xu, X. Hao, Z. Wang and L. Chen, J. Mater. Chem. A, 2015, 3, 7849–7854 CAS
.
- S. Wenzel, T. Hara, J. Janek and P. Adelhelm, Energy Environ. Sci., 2011, 4, 3342–3345 CAS
.
- E. Irisarri, A. Ponrouch and M. R. Palacin, J. Electrochem. Soc., 2015, 162, A2476–A2482 CrossRef CAS
.
- K. Tang, L. Fu, R. J. White, L. Yu, M. M. Titirici, M. Antonietti and J. Maier, Adv. Energy Mater., 2012, 2, 873–877 CrossRef CAS
.
- L. Fu, K. Tang, K. Song, P. A. van Aken, Y. Yu and J. Maier, Nanoscale, 2014, 6, 1384–1389 RSC
.
- Y. Fang, D. Gu, Y. Zou, Z. Wu, F. Li, R. Che, Y. Deng, B. Tu and D. Zhao, Angew. Chem., Int. Ed., 2010, 49, 7987–7991 CrossRef CAS PubMed
.
- Y. Fang, Y. Lv, R. Che, H. Wu, X. Zhang, D. Gu, G. Zheng and D. Zhao, J. Am. Chem. Soc., 2013, 135, 1524–1530 CrossRef CAS PubMed
.
- Z. Wang, L. Qie, L. Yuan, W. Zhang, X. Hu and Y. Huang, Carbon, 2013, 55, 328–334 CrossRef CAS
.
- D. Xu, C. Chen, J. Xie, B. Zhang, L. Miao, J. Cai, Y. Huang and L. Zhang, Adv. Energy Mater., 2016, 6, 1501929 CrossRef
.
- Y. Xue, J. Liu, H. Chen, R. Wang, D. Li, J. Qu and L. Dai, Angew. Chem., Int. Ed., 2012, 51, 12124–12127 CrossRef CAS PubMed
.
- J. Xu, M. Wang, N. P. Wickramaratne, M. Jaroniec, S. Dou and L. Dai, Adv. Mater., 2015, 27, 2042–2048 CrossRef CAS PubMed
.
- L. Ji, Z. Lin, A. J. Medford and X. Zhang, Carbon, 2009, 47, 3346–3354 CrossRef CAS
.
- X. Zhou and Y. G. Guo, ChemElectroChem, 2014, 1, 83–86 CrossRef
.
- F. Su, C. K. Poh and J. S. Chen, et al., Energy Environ. Sci., 2011, 4, 717–724 CAS
.
- S. X. Wang, S. Chen, Q. Wei, X. Zhang, S. Y. Wong, S. Sun and X. Li, Chem. Mater., 2014, 27, 336–342 CrossRef
.
- Y. G. Guo, J. S. Hu and L. J. Wan, Adv. Mater., 2008, 20, 2878–2887 CrossRef CAS
.
- M. Yoshio, H. Wang and K. Fukuda, Angew. Chem., Int. Ed., 2003, 115, 4335–4338 CrossRef
.
| This journal is © The Royal Society of Chemistry 2016 |