 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dissociative adsorption of O2 on negatively charged nitrogen-doped single-walled carbon nanotubes: first-principles calculations†
Divya
Srivastava‡
* and
Kari
Laasonen
*
Department of Chemistry, Aalto University, P. O. Box 16100, 00076 Aalto, Finland. E-mail: divya@iisermohali.ac.in; kari.laasonen@aalto.fi
First published on 1st September 2016
Abstract
Spin unrestricted density functional theory (DFT) calculations have been used to investigate the molecular and dissociative adsorption of O2 on achiral substitutional nitrogen-doped single-walled carbon nanotubes (N-SWNTs) with and without additional charges. Adsorption (dissociation) of O2 on the charged tubes is quite complex. The N-SWNTs with an additional electron with different orientations exhibit different behaviors. The armchair N-SWNTs carrying an extra electron have lower chemisorption (up to 0.6 eV) and reaction (up to 1.2 eV) energies for O2 compared to the corresponding neutral N-SWNTs. For (10, 10) N-SWNTs, the dissociation barrier decreases from 1.36 eV (in neutral) to 0.76 eV (in negatively charged). The reactivity of the armchair N-SWNTs with low nitrogen content can be increased by adding extra electrons. For the metallic zigzag N-SWNTs, an additional electron affects the O2 adsorption and dissociation marginally. However, negatively charged zigzag N-SWNTs, which are semiconducting when un-doped, have significantly lower O2 adsorption, reaction and dissociation barrier energies than the neutral N-SWNTs. We also studied the molecular and dissociative adsorption of O2 on (10, 10) single-walled nanotubes doped with two substitutional nitrogen atoms (N2-SWNTs), as a function of extra charge. The chemisorption and reaction energies decrease linearly with the increasing number of extra electrons. The barrier for O2 dissociation on N2-SWNTs is found to increase with the increasing number of additional electrons. Our results point out that the dissociative adsorption of an oxygen molecule on N-SWNTs depends on many parameters: curvature, chirality, and charge. The effect of additional charge on the reactivity of the N-doped SWNTs is rather independent of the tube diameter, but depends on the tube orientation.
Introduction
Fuel cells are one of the most promising power sources, producing electricity by electrochemical conversion of hydrogen and oxygen into water. Unfortunately, the low rate of the oxygen reduction reaction (ORR) at the cathode causes performance loss. To enhance the performance of a fuel cell, one needs to use a suitable electrocatalyst which speeds up the sluggish ORR. But good catalysts such as platinum and its alloys are very expensive, thus forestalling the use of the fuel cell for most practical applications. Therefore, wide efforts are being made to find efficient and low-cost electrocatalysts to replace the expensive noble metal platinum in fuel cells. In this regard, nitrogen-doped carbon nanomaterials (N-doped CNMs) such as nanotubes, nanoribbons and graphene, are attractive metal-free electro-catalysts, as they are inexpensive and have long-term operation stability.1–12 N-doped CNMs show outstanding catalytic activity towards ORR in acidic and alkaline electrolyte media.2,5–8 Electron rich N-doping effectively modifies the chemical and electronic properties of N-doped CNMs. N is more electronegative than carbon (C), therefore the C–N bond is strongly polarized towards the N. The partial positively charged C atoms adjacent to N help in breaking the O–O bond of the oxygen molecule.The mechanism of ORR on different nitrogen chemical states in N-doped CNMs is poorly understood. In many studies it has been proposed that the catalytic activity in N-doped CNMs is attributed to nitrogen in the form of pyridinic and pyrrolic/pyridone-type nitrogen groups.2,3,10 However, Niwa et al. found that N-doped CNTs with a greater number of graphite-like nitrogen possess a higher ORR activity than those with a relatively larger amount of pyridine-like nitrogen.11 Theoretical studies have also supported these results. Ikeda et al.,13 observed in their simulation that ORR activity in N-doped carbon alloy catalysts is related to the presence of graphite-like nitrogen. Presence of higher concentration of graphite-like N is more important for O2 dissociation in N-doped single-walled nanotubes (SWNTs).14,15
Various research group performed theoretical calculations3,14–17 on very narrow nitrogen-doped single-walled nanotubes (N-doped SWNT) (diameter less than 1 nm). However, the carbon nanotubes used in ORR study are either few-walled or multi-walled with diameters in the range of 1 to 100 nm.2,5,7–9,12 In our previous study we have found that dissociation of an oxygen molecule on single-walled carbon nanotubes with substitutional N-doping depends on number of parameters such as diameter, metallicity and chirality. Nevertheless, tube diameter is a key parameter.15 The oxygen dissociation energy barrier is substantially lower on the narrow N-SWNT (diameter < 1 nm) comparing to that of on the tube with diameter greater than 1 nm. In all these studies, the activation barrier for oxygen dissociation was calculated on N-SWNTs with type of nitrogen functional groups and defects.14–16
In electrochemical process, presence of extra electrons is very important for the reactions to occur. ORR involves transfer of electrons. In fuel cell, N-doped SWNTs are used as catalyst to enhance the kinetics of the ORR, the charged N-doped SWNT may play an important role in further improving the catalytic activity and hence reaction kinetics. However, the charged N-doped SWNT seems to have been less studied, especially how additional charges affect O2 dissociative adsorption over N-doped SWNT.
In this work, we investigate the effect of extra electron in substitutional N-doped SWNTs on oxygen molecular and dissociative adsorption. In substitutional N-doping, a carbon (C) atom is removed and replaced by a nitrogen (N) atom. N-SWNT is an abbreviation for substitutional nitrogen-doped single walled carbon nanotube(s). To this end, zigzag ((n, 0), where n = 15 to 21) and armchair ((n, n), where n = 9 to 12) N-SWNTs of diameter greater than 1 nm are used. Added an extra electron in N-SWNTs is small compared to the total number of electrons in neutral N-SWNTs (more than a thousand) and so is the change in total electronic charge density of the system. In a recent report, Mahshid Rahimi et al. used a combination of molecular dynamics and grand canonical Monte Carlo to study the CO2 adsorption on charged SWNT arrays. They used fixed charge of 0.01 to 0.04 e on each C atom to investigate the charge effect.18 Experimentally investigated K and Cs doped SWNTs showed the excess negative charge of about 0.125 e per C atom.19 In our study, charged N-SWNTs have extra charge of ∼0.004 to 0.01 e per C atom. For the N2-SWNTs, the excess charges are in the range of 0.005 to 0.02 e per C atom.
Computational details and methods
In this paper, all spin-polarized density functional theory (DFT) calculations are carried out with CP2K/QUICKSTEP code,20 which is based on the Gaussian and plane waves (GPW) method. The exchange–correlation functional has been treated by the generalized gradient approximation (GGA) formulated by Perdew, Burke and Ernzerhof (PBE).21 Norm-conserving Goedecker–Teter–Hutter (GTH)22 pseudo-potentials were employed. A plane wave cutoff of 500 Ry was used for the finest level of the multi-grid. Broyden–Fletcher–Goldfarb–Shanno algorithm (BFGS) method is used for the geometry optimization and system was relaxed until forces were less than 0.023 eV Å−1. The N-SWNTs used in this study are open-ended (n, 0) with n = 15 to 21 for the zigzag and (n, n) with n = 9 to 12 for the armchair. Length of the unit cell (L) along the tube axis direction for (n, 0) and (n, n) tubes are 12.78 Å and 12.298 Å, respectively. The climbing image nudge elastic band (CI-NEB)23 method was used to compute the minimum energy path (MEP) and the activation energy barriers for oxygen dissociation on N-SWNTs. Eight images were interpolated between the initial and final states to describe a MEP. To develop the understanding of O2 dissociation on N-doped carbon nanotubes is of fundamental importance to electrolysis. Dissociation of an oxygen molecule on N-doped nanotube proceeds by precursor states that is a physisorbed state and a chemisorbed state. In physisorbed state, O2 adsorption is mediated by weak forces (see Fig. 1(a)). O2 molecule remains in its triplet state and can diffuse along the surface to find likely site for chemisorption. In chemisorbed state, O2 molecule forms covalent bond with carbon atoms of the nanotube and loses its magnetic moment (see Fig. 1(b)). Dissociative adsorption often begins with chemisorption precursor state and adsorbed oxygen molecule dissociates in to two oxygen atoms (see Fig. 1(c)). It is important to note that in this study we have only considered the adsorption of an oxygen molecule on axial C–C site, adjacent to nitrogen atom (see Fig. 1). From previous studies, we have found that the axial C–C site is slightly more energetically favorable for O2 adsorption than the circumferential C–C site.15,16As a first step for O2 adsorption (physisorption state) on both charged and neutral N-SWNTs, we put an oxygen molecule on top of the axial C–C bond at a distance 3.0 Å. We keep orientation of the O–O bond parallel to C–C bond and performed an ionic minimization of the N-SWNT + O2 system in order to find out the optimized structure. Second step for O2 chemisorption, we decreased the distance between the oxygen molecule and the nanotube. We placed the O2 molecule near the tube surface at a distance of around 1.5 Å and relaxed the system (only ions are allowed to move). In relaxed structure, oxygen molecule forms covalent bonds with the C atoms of the tube (see Fig. 1(b)). And O–O bond length increases from its gas phase value of 1.23 Å to ∼1.5 Å. Finally, for dissociative adsorption, oxygen atoms are keeping on opposite C–C bridge sites of the tube axis (see Fig. 1(c)) and optimized the systems. Shan et al. has observed lowest activation barrier for this type of dissociation on (10, 0) SWNT and N-SWNT.16 Physisorption, chemisorption and reaction energies of the N-SWNT + O2 systems are calculated by using the following equation:
| EX= E(N-SWNT + O2)X − E(N-SWNT) − EO2 | (1) |
The activation barrier must be overcome in order for an oxygen molecule to go from one state to another. The activation barrier is only calculated for dissociative adsorption. NEB calculations have been performed to find MEP for an oxygen molecule going from chemisorption state (an initial state, (IS)) to dissociation state (a final state (FS)). The potential energy maximum along MEP is the transition state (TS). The MEP profile is shown in Fig. 2. The activation barrier energy is calculated with respect to the reference energy of the physisorption state.
It is well established that GGA does not describe very well the weak interaction such as van der Waals interactions. However, our main concern is to calculate activation barrier energy for dissociative adsorption. We investigate the molecular and dissociative adsorption of O2 on negatively charged (10, 10) N-SWNT with and without DFT-D3 van der Waals (vdW) correction. We observe that vdW yields lower adsorption and reaction energy compared to the conventional GGA results, while the O2 dissociation barrier remains almost unchanged. The barrier is 0.76 eV for GGA and 0.74 eV for GGA with vdW. Both GGA and GGA + vdW gave comparable results; hence, we have used the conventional GGA for our study.
Results and discussions
In the following, we present the molecular and dissociative adsorption of an oxygen molecule on neutral and charged N-SWNTs. Luo Ji et al.24 observed that the total energy of the nanotube is a quadratic function of the amount of extra charge. With increasing the number of electrons the total energy first decreases, and then increases after a minimum is reached. Total energy of SWNT carrying small negative charges (up to 5 extra electrons) may have a lower total energy than that neutral one.24 First, we studied the molecular and dissociative adsorption of O2 molecule on (10, 10) N-SWNT, carrying an extra charge Q (N-SWNTQ). Where, Q is ranging from −1 to +1. It is clear from the adsorption energies, summarized in Table 1, that N-SWNT with an extra electron has lower physisorption, chemisorption and reaction energies than the neutral and positively charged N-SWNTs. Therefore, we have considered O2 adsorptive dissociation on neutral and negatively charged N-SWNTs.| N-SWNTQ (10, 10) MA | Q = −1 | Q = 0 | Q = +1 |
|---|---|---|---|
| Physisorption energy (eV) | −0.136 | −0.10 | −0.037 |
| Chemisorption energy (eV) | 0.287 | 0.885 | 1.45 |
| Reaction energy (eV) | −0.794 | 0.449 | 1.26 |
Calculated O–O, C–O, C*–O, C–C*, and C*–N bond distances for metallic zigzag (MZ) ((15, 0), (18, 0), and (21, 0)), semi-conducting zigzag (SZ) ((16, 0), (17, 0), (19, 0) and (20, 0)), and metallic armchair (MA) ((n, n), n = 9 to 12) N-SWNTs(Q=−1 and 0) are given in Table S.†25 Here C* is one of the carbon atoms adjacent to N atom, on which adsorption of an oxygen molecule is considered. From Table S,†25 we can see that in physisorbed state the equilibrium distance between O2 and the N-SWNT(Q=−1 and 0) is more than 3 Å, which indicates that a weak interaction exists between them. The optimized O–O bond length is in the range of 1.24 Å to 1.28 Å, which is larger than the O–O bond length 1.23 Å in gas phase. This indicates that the O–O bonding is slightly weekend when the O2 is physisorbed on the tube. In this state the oxygen molecule has a non-zero spin magnetic moment, which indicates that the molecule is in triplet state. In some of the negatively charged N-SWNTs, O2 prefers to be on top of the C*–N site instead of the C–C* site. It can be inferred from the data presented in Table S†25 that extra electron has marginal effect on the N-SWNTs structure. In the chemisorbed state, O2 molecule prefers to bind perpendicular to the C–C* site of the N-SWNTs(Q=−1 and 0) in tilted configuration. The C*–O bond length (1.47 to 1.50 Å) is shorter than the C–O bond length (1.52 to 1.59 Å). The C–C* bond distance on which O2 molecule is chemisorbed is in the range of 1.53 to 1.54 Å for the zigzag N-SWNTs(Q=−1 and 0) and 1.56 to 1.57 Å for the armchair N-SWNTs(Q=−1 and 0). For all the considered tubes, the bond length of C*–N is in the range of 1.46 to 1.48 Å. From Table S,†25 the O–O bond length is elongated from 1.23 Å/1.24 to 1.28 Å in the ground/physisorbed state to 1.49 to 1.50 Å in the chemisorbed state and O2 molecule has a zero spin magnetic moment. A transition from the triplet state of O2 molecule to the singlet state of the chemisorbed O2 molecule is observed. The O–O bond-length in the dissociative adsorption is larger than 2.5 Å for all the studied N-SWNTs(Q=−1 and 0). It indicates that on the surface of tube O2 dissociates to form adsorbed oxygen atoms. In the dissociative adsorption, C–C* and C*–N bond-lengths for (n, 0) and (n, n) type N-SWNTs(Q=−1 and 0) are varying from 1.49 to 1.57 Å and 1.46 to 2.10 Å, respectively. The N-SWNTs(Q=−1 and 0) with C–N distance greater than 1.6 Å implying that the C–N bond would break. N atom transforms from the graphitic-like configuration to the pyridinic configuration. In this case as shown in Fig. 3, one of the oxygen atoms prefers to bind with C* atom, with C*–O bond-length 1.24 to 1.28 Å, instead of C–C bridge site (see Fig. 1(c)) and the other oxygen atom dissociates along C–C bridge site. The calculated reaction energy for such configurations is less than 0.15 eV (see Table 2). The C–C* and C*–N bond lengths of the N-SWNT(Q=−1 and 0) are increased in the dissociate (chemisorbed) state from their corresponding values in the physisorbed state. This indicates that a structural change occurred due to O2 dissociation (chemisorption).
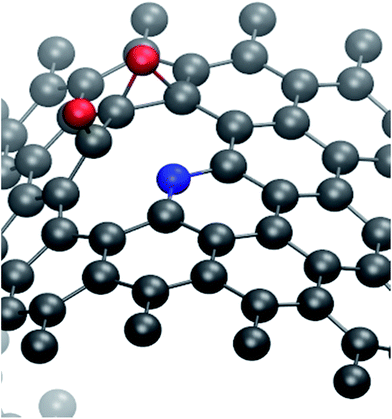 | ||
| Fig. 3 Optimized structure of O2 dissociative adsorption on N-SWNT. One of the O atoms adsorbed on C site rather than on C–C site (see Fig. 1(c)) and C–N bond breaks. | ||
| N-SWNTQ | Physisorption energy | Chemisorption energy | Reaction energy | Activation barrier | ||||
|---|---|---|---|---|---|---|---|---|
| Charge (Q) | 0 | −1 | 0 | −1 | 0 | −1 | 0 | −1 |
| (15, 0)MZ | −0.007 | −0.056 | 0.673 | 0.584 | 0.30 | 0.212 | 1.1 | 1.0 |
| (18, 0)MZ | 0.0007 | −0.033 | 0.837 | 0.762 | 0.135 | −0.001 | — | — |
| (21, 0)MZ | −0.0042 | 0.0067 | 0.948 | 0.905 | 0.529 | 0.483 | — | — |
| (16, 0)SZ | −0.058 | −0.065 | 0.832 | 0.518 | 0.095 | −0.276 | 2.22 | 1.24 |
| (17, 0)sz | 0.004 | −0.015 | 0.850 | 0.584 | 0.153 | −0.244 | — | — |
| (19, 0)sz | 0.007 | −0.008 | 0.999 | 0.718 | 0.579 | 0.462 | 1.44 | 1.16 |
| (20, 0)SZ | −0.015 | −0.034 | 0.976 | 0.738 | 0.642 | 0.484 | 1.47 | 1.20 |
| (9, 9)MA | −0.157 | −0.181 | 0.76 | 0.154 | 0.173 | −0.932 | — | — |
| (10, 10)MA | −0.100 | −0.136 | 0.885 | 0.287 | 0.449 | −0.794 | 1.36 | 0.76 |
| (11, 11)MA | −0.111 | −0.182 | 0.934 | 0.359 | 0.504 | −0.699 | — | — |
| (12, 12)MA | −0.105 | −0.154 | 0.947 | 0.388 | 0.375 | −0.651 | — | — |
To investigate the effect of charge on O2 dissociation, we have calculated the physisorption, chemisorption and reaction energies for both neutral and charged tubes (N-SWNTs(Q=−1 and 0)) by using eqn (1). We have also calculated the activation energy barrier for O2 dissociation on some of the studied N-SWNTs(Q=−1 and 0). The physisorption, chemisorption, reaction energies and activation barriers for studied N-SWNTs(Q=−1 and 0) are tabulated in Table 2. Here reported values of chemisorption, and reaction for N-SWNTs are different from the values reported in our previous paper.15 This is because we have used binding energy (=E(N-SWNT) + E(O2)) as a reference energy in this paper instead of physisorption energy, as had been used in the previous paper. For (16, 0) N-SWNT we have observed different values than the previously reported values. The activation barrier is calculated with respect to reference energy of the physisorption state (see Fig. 2).
In Fig. 4(a) the chemisorption and reaction energies of (n, 0)-type N-SWNTs (Q=−1 and 0) are plotted with respect to diameter of tubes. MZ N-SWNTs ((n, 0) n = 15, 18, and 21) carrying an extra electron have slightly lower chemisorption and reaction energies than the corresponding neutral metallic N-SWNTs. Thus, an extra electron on metallic (n, 0) N-SWNTs has a weak effect on the oxygen molecular and dissociative adsorption. We have calculated oxygen dissociation energy barrier for metallic (15, 0) N-SWNT(Q=−1 and 0). The difference in the activation energy barrier between the neutral and charged (15, 0) N-SWNT is 0.10 eV, which is very small. The SZ N-SWNTs with an extra electron have significantly lower chemisorption and reaction energies than the corresponding neutral N-SWNTs (see (16, 0), (17, 0), (19, 0) and (20, 0) in Fig. 4(a)). However, the effect of an additional electron on O2 molecular and dissociative adsorption energy becomes weaker and weaker as the tube diameter increases. This is because the energy band-gap of the SWNT is approximately inversely proportional to the tube diameter. From Table 2, it can be seen that these charged tubes have significantly smaller activation energy barriers than their neutral counterparts. In Fig. 4(b), chemisorption and reaction energies for (n, n) type N-SWNTs(Q=−1 and 0) are plotted against the tube diameter. The chemisorption and reaction energies are varying almost linearly with the diameter of the N-SWNTs (Q=−1 and 0) except the reaction energy for N-SWNTs(Q=0).
It is noteworthy that the chemisorption and reaction energies in the armchair N-SWNTs(Q=−1) are reduced by more than 0.5 eV and 1.0 eV, respectively, when compared to N-SWNTs(Q=0). Fig. 4(b) clearly shows that the effect of an additional electron on the O2 adsorption and reaction energies is rather independent of the tube diameter. We have calculated the O2 dissociation barrier for both neutral and negatively charged (10, 10) N-SWNT, listed in Table 2. Addition of an extra electron on (10, 10) N-SWNT lowered the O2 dissociation barrier by 0.6 eV. Our calculations show that O2 dissociative adsorption is more favorable on the negatively charged armchair N-SWNT than the neutral armchair N-SWNT.
We recalculate the adsorption of O2 molecule on (10, 10), (16, 0) and (15, 0) N-SWNTs(Q=−1 and 0) for unit cell of double length along the tube axis direction. We observed similar behavior. The adsorption energies decrease when we add an electron in (10, 10) and (16, 0) N-SWNTs, while there is very negligible effect of an extra electron in the adsorption energies for the (15, 0) N-SWNT. For (16, 0) and (10, 10) N-SWNTs, O2 adsorption is also examined on the tubes with two extra electrons. The calculated chemisorption and reaction energies for (16, 0) N-SWNT(Q=−2) are 0.474 eV and −0.379 eV, respectively. For (10, 10) N-SWNTs the chemisorption and reaction energies are 0.169 eV and −0.956 eV, respectively. The adsorption and reaction energies of N-SWNT(Q=−2) are slightly lower than those of the N-SWNT(Q=−1) (see Table 2), therefore are not considered further. These results show that negatively charged metallic armchair N-SWNT has lower chemisorption and reaction energies than the neutral N-SWNT. However, for the metallic zigzag N-SWNTs exhibited similar adsorption energies for both neutral and negatively charged. In order to explain these findings, we have performed the Bader charge26–28 and local density of states (LDOS) analysis. Atomic charge values of N atom and C atoms adjacent to N atom for N-SWNT(Q=−1 and 0) are reported in Table 3. Our Bader analysis shows that the 2p electrons of the C atoms are being transferred to the adjacent N atom, which gives a net positive charge on C atoms and a net negative charge on the N atom. The atomic charge values for C next to N and N atoms are comparable with previously reported values in the ref. 15, 29 and 30 and are larger than those reported by S. Ni et al.14 But this is of little concerns for us since we are interested in to see the effect of additional electron on charge transfer. Therefore, we are not addressing this issue here. Table 3 reveals that atomic charges on N and C atoms in neutral and charged N-SWNTs are almost similar. This is true for both zigzag and armchair tubes. Thus, we can conclude that an extra electron is delocalized and distributed uniformly throughout the tubes. On the contrary the (16, 0) tube shows dissimilar behavior. The absolute atomic charge on N (one of the C atoms) in the negatively charged tube is lower than the neutral tube. This may be due to the localization of extra electron in the vicinity of the C–N bond which prohibits the electrons transfer from the C to N. We also examine the local density of states (LDOS) of C and N for these studied tubes and shown in Fig. 5 for (15, 0) and (16, 0) zigzag, and in Fig. 6 for (10, 10) armchair tubes. The LDOS of C and N for other studied tubes are shown in Fig. S1†25 for zigzag and Fig. S2†25 for arm-chair tubes. The left and the right panels of the Fig. 5 (Fig. S1†)25 and Fig. 6 (Fig. S2†)25 show the LDOS of C atoms and N atom, respectively. The black and blue dotted curves represent the LDOS of N-SWNT(Q=0) and N-SWNT(Q=−1), respectively. It can be seen from Fig. 5 that both charged and neutral (n, 0) type metallic N-SWNTs have almost similar LDOS, therefore have almost similar chemisorption and reaction energies. Whereas the (n, n) type N-SWNTs (Q = −1) have more states above and in the vicinity of the Fermi level than those of the neutral N-SWNTs (see Fig. 6). The increase in LDOS of C and N near and above the Fermi level enhances the chemical reactivity of the tubes, facile the molecular and dissociative adsorption of an oxygen molecule. O2 chemisorption and reaction energies decrease, therefore the activation barrier energy.
| Zigzag N-SWNT | Armchair N-SWNT | |||||
|---|---|---|---|---|---|---|
| Q = 0 | Q = −1 | Q = 0 | Q = −1 | |||
| N | (15, 0) | −2.5 | −2.5 | (9, 9) | −2.75 | −2.7 |
| C | 0.83 | 0.84 | 0.87 | 0.84 | ||
| C | 0.73 | 0.72 | 0.87 | 0.84 | ||
| C | 0.72 | 0.71 | 0.86 | 0.81 | ||
| N | (16, 0) | −2.53 | −2.00 | (10, 10) | −2.19 | −2.1 |
| C | 0.82 | 0.29 | 0.89 | 0.91 | ||
| C | 0.80 | 0.77 | 0.89 | 0.87 | ||
| C | 0.73 | 0.73 | 0.33 | 0.31 | ||
| N | (17, 0) | −2.59 | −2.59 | (11, 11) | −1.72 | −1.64 |
| C | 0.85 | 0.84 | 0.44 | 0.39 | ||
| C | 0.74 | 0.72 | 0.43 | 0.39 | ||
| C | 0.79 | 0.78 | 0.72 | 0.69 | ||
| N | (18, 0) | −2.64 | −2.66 | (12, 12) | −2.67 | −2.61 |
| C | 0.84 | 0.83 | 0.94 | 0.91 | ||
| C | 0.77 | 0.93 | 0.93 | 0.92 | ||
| C | 0.72 | 0.73 | 0.85 | 0.74 | ||
| N | (19, 0) | −2.68 | −2.66 | |||
| C | 0.93 | 0.88 | ||||
| C | 0.83 | 0.80 | ||||
| C | 0.83 | 0.84 | ||||
| N | (20, 0) | −2.64 | −2.66 | |||
| C | 1.01 | 1.0 | ||||
| C | 0.85 | 0.86 | ||||
| C | 0.76 | 0.75 | ||||
| N | (21, 0) | −2.54 | −2.54 | |||
| C | 0.81 | 0.82 | ||||
| C | 0.77 | 0.74 | ||||
| C | 0.73 | 0.74 | ||||
 | ||
| Fig. 5 Local density of states of zigzag N-SWNTs(Q=−1 and 0) (a) all C atoms, and (b) a N atom. EF represents the Fermi level. | ||
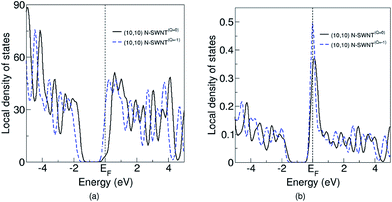 | ||
| Fig. 6 Local density of states of armchair N-SWNTs(Q=−1 and 0) (a) all C atoms, and (b) a N atom. EF represents the Fermi level. | ||
It is well established that nitrogen concentration in nitrogen-doped carbon materials plays an important role for catalytic activity. We therefore investigate the effect of charge on (10, 10) SWNT with two graphitic-like nitrogen dopants (N2-SWNT), shown in Fig. 7. We consider this configuration because in our previous study we have observed lowest adsorption energies and activation barrier for this configuration.15
O2 adsorption is considered on (10, 10) N2-SWNT carrying extra charge Q ranging from −4 to 1. It is important to note that O2 molecule directly goes to chemisorbed state on negatively charged N2-SWNT. However, O2 physisorbed on N2-SWNT(Q=0 and 1) and N-SWNT(Q= −1 and 0). Fig. 8 illustrates variation in the chemisorption and reaction energies with respect to extra charge. The negative adsorption and reaction energies indicate that the O2 molecular adsorption and dissociation are exothermic process. The chemisorption and reaction energies decrease linearly with increasing extra negative charges on the (10, 10) N2-SWNT. Which means adding extra electrons can promote O2 adsorption (chemisorption and dissociation) on the N2-SWNT. We have found an opposite effect (increase of chemisorption and reaction energies) when O2 molecule adsorbed on the N2-SWNT carrying an extra positive charge. Comparing O2 adsorption energies of this positively charged tube with the (10, 10) N-SWNT(Q=−1 and 0), the O2 chemisorption and reaction energies of N2-SWNT(Q=+1) are lower than those of N-SWNT(Q=0) and higher than those of the N-SWNT(Q=−1).
 | ||
| Fig. 8 The chemisorption energy and the reaction energy are plotted against the additional charge in the (10, 10) N2-SWNT. | ||
The calculated activation barrier for O2 dissociation on N2-SWNT(Q=0), N2-SWNT(Q=−1) and N2-SWNT(Q=−2) is 0.15 eV, 0.33 eV and 0.42 eV, respectively. The increase in activation barrier on negatively charged N2-SWNT can be attributed to the fact that the strong chemisorption of O2 on the tube makes O2 dissociation less favorable. Note, we have not observed physisorption of O2 on negatively charged N2-SWNT tube. Therefore, the activation barrier for these tubes are calculated with respect to the reference energy of the chemisorption states.
To gain further insight we performed the Bader26–28 and LDOS analysis on the (10, 10) N2-SWNTs(Q=−4 to 1). Table 4 shows net atomic charge on N atoms and C atoms adjacent to one or two N atoms (see Fig. 7(b)) of (10, 10) N2-SWNT with and without extra charge. Each N atom carries a negative partial charge and each C next to N has a partial positive charge. This shows that partial electron charges are transferred from the adjacent C atoms to the more electronegative N atoms. The carbon atom (C1), bonded with two N atoms, has almost three times more positive atomic charge than the C adjacent to a N atom. So N-doping in SWNT causes non-uniform distribution of atomic charge around the N.
| Q | 1 | 0 | −1 | −2 | −3 | −4 |
|---|---|---|---|---|---|---|
| N1, N2 | −2.69, −2.55 | −2.64, −2.5 | −2.63, −2.48 | −2.64, −2.49 | −2.52, −2.67 | −2.57, −2.77 |
| C1 | 2.14 | 2.21 | 2.14 | 2.12 | 2.0 | 2.04 |
| C11, C12 | 0.70, 0.72 | 0.70, 0.59 | 0.61, 0.67 | 0.64, 0.63 | 0.65, 0.68 | 0.65, 0.68 |
| C21, C22 | 0.70, 0.72 | 0.70, 0.59 | 0.67, 0.66 | 0.64, 0.64 | 0.68, 0.65 | 0.64, 0.68 |
It is clear from Table 4 that there is no significant atomic charge rearrangement in the N2-SWNT when extra charges are added. Therefore, the extra charge(s) is (are) uniformly distributed over the tube. Fig. 9 illustrates the LDOS of C and N atoms for the N2-SWNT carrying Q (=−4 to 1) extra charge. It is clearly shown that the Fermi energy level is shifted toward the conduction band when electrons are added in the neutral N2-SWNT. And it moves toward the valence band for positively charged N2-SWNT. The change in LDOS of negatively charged systems with increasing extra electrons (Q = −1 to −4) is marginal. This might be related to the fact that the number of extra electrons is negligibly small compare to the total number of electrons in the tubes (802 electrons).
 | ||
| Fig. 9 Local density of states of (10, 10) N2-SWNTs(Q=−4 to 1) (a) all C atoms, and (b) two N atoms. EF represents the Fermi energy level. | ||
The negatively charged N2-SWNT has stronger LDOS peak for C and N at Fermi level as compared to the neutral N2-SWNT and the positively charged N2-SWNT. However, the intensity of the LDOS peaks for C and N in the vicinity of the Fermi level is higher for the neutral tube than the positively charged tube. Therefore, the chemical reactivity of the N2-SWNT is increased after addition of extra electrons and reactivity decreases when an electron is removed from the tube. It is reported that N-doping reduces the work function of the SWNT.14 We have calculated work function of the N2-SWNT(Q=−1 to 1). It is important to note that adding an extra electron to the tube causes an up-shift in the Fermi level of the tube, while the Fermi level decreases for positively charged tube. Therefore, the work function of the positively (negatively) charged tube is increased (decreased) by more than 1.0 eV (about 1.0 eV) compared to the neutral tube. Consequently, an increase in local density of states around Fermi level and reduction in work function in the negatively charged N2-SWNT facilitates charge transfer to the adsorbed oxygen molecule, which lowers the adsorption and reaction energies.
Conclusions
In order to provide fundamental understanding on how extra charges can affect the dissociative adsorption of O2 molecule on N-doped SWNTs, spin polarized DFT calculations have been performed. The adsorption of O2 molecule on the negatively charged zigzag N-SWNTs(Q=−1) is found to be very similar to the neutral zigzag N-SWNTs. Zigzag N-SWNTs carrying an extra electron have approximately same chemisorption and reaction energies as those of the neutral zigzag N-SWNTs, except those which are semiconducting in their pristine form. We have observed significant reduction in adsorption energies and energy barrier for these negatively charged zigzag N-SWNTs. For N-SWNT with armchair orientation, the adsorption and dissociation of O2 molecule is energetically more favorable on negatively charged than the neutral. The calculated O2 dissociation energy barrier for (10, 10) N-SWNT with an extra electron is 0.76 eV, which is about 0.6 eV less in energy than that of neutral (10, 10) N-SWNT. Our results indicate that oxygen dissociation energy barrier is related to its adsorption and reaction energies. Energy barrier can be reduced by lowering the adsorption and reaction energies. We also examine the effect of Q (=−4 to 1) extra charge on (10, 10) N2-SWNT for a particular configuration in which one of the C atoms is bonded with two N atoms and a C atom. Our calculations show that the adsorption of O2 is energetically favorable on N2-SWNT carrying Q extra electrons while it is less favorable on positively charged N2-SWNT. Extra electrons in N2-SWNT improve chemical reactivity and reduce its work function, which makes chemisorption of O2 more thermodynamically stable. Therefore, the dissociation of an oxygen molecule becomes less favorable on N2-SWNT with additional electron(s), compared to the neutral N2-SWNT. Our findings indicate that an extra electron has an effect on the electrochemical reactivity of N-SWNTs but in rather complex way. The presence of an extra electron enhances the chemical reactivity in armchair configuration while it remains unaffected for metallic zigzag orientation. Thus the effect of extra negative charge on the reactivity of the N-SWNT is a function of the tube orientation. And O2 dissociation barrier can be reduced for armchair SWNTs with low nitrogen concentration, by making the tube negatively charged.Acknowledgements
This work was supported by Academy of Finland Centre of Excellence function by COMP. We would like to thank Dr Tanja Kallio for useful discussions of the N-CNT usage in fuel cells. Divya Srivastava thanks DST, India for INSPRE faculty award (DST/INSPIRE/04/2015/000579). Divya Srivastava also thanks CSC-IT Center for Science, for providing high-performance computing facilities.Notes and references
- S. Maldonado and K. J. Stevenson, J. Phys. Chem. B, 2005, 109, 4707 CrossRef CAS PubMed.
- S. Kundu, T. C. Nagaiah, W. Xia, Y. Wang, S. V. Dommele, J. H. Bitter, M. Santa, G. Grundmeier, M. Bron and W. Schuhmann, et al. , J. Phys. Chem. C, 2009, 113, 14302 CAS.
- K. Gong, F. Du, Z. Xia, M. Durstock and L. Dai, Science, 2009, 323, 760 CrossRef CAS PubMed.
- L. T. Qu, Y. Liu, J. B. Baek and L. M. Dai, ACS Nano, 2010, 4, 1321 CrossRef CAS PubMed.
- H. Li, H. Liu, Z. Jong, W. Qu, D. Geng, X. Sun and H. Wang, Int. J. Hydrogen Energy, 2011, 36, 2258 CrossRef CAS.
- L. Zhang and Z. Xia, J. Phys. Chem. C, 2011, 115, 11170 CAS.
- Z. Chen, D. Higgins and Z. Chen, Carbon, 2010, 48, 3057 CrossRef CAS.
- J. Vazquez-Arenas, D. Higgins, Z. Chen, M. Fowler and Z. Chen, J. Power Sources, 2012, 205, 215 CrossRef CAS.
- C. Wnag, M. Waje, M. J. Tang, C. R. Haddon and Y. Yan, Nano Lett., 2004, 4, 345 CrossRef.
- V. C. Rao, R. C. Cabrera and Y. Ishikawa, J. Phys. Chem. Lett., 2010, 1, 2622 CrossRef.
- H. Niwa, K. Horiba, Y. Harada, M. Oshima, T. Ikeda, K. Terakura, J. Ozaki and S. Miyata, J. Power Sources, 2009, 187, 93 CrossRef CAS.
- T. Sharifi, G. Hu, X. Jia and T. Wågberg, ACS Nano, 2012, 6, 8904 CrossRef CAS PubMed.
- T. Ikeda, M. Boero, S.-F. Huang, K. Terakura, M. Oshima and J.-ichi Ozaki, J. Phys. Chem. C, 2008, 112, 14706 CAS.
- S. Ni, Z. Li and J. Yang, Nanoscale, 2012, 4, 1184 RSC.
- D. Srivastava, T. Susi, M. Broghei and L. Kari, RSC Adv., 2014, 4, 15225 RSC.
- B. Shan and K. Chao, Chem. Phys. Lett., 2010, 492, 131 CrossRef CAS.
- P. Zhang, S. J. Lian and Q. Jiang, Phys. Chem. Chem. Phys., 2012, 14, 11715 RSC.
- M. Rahimi, J. K. Singh and F. Müller-Plathe, J. Phys. Chem. C, 2015, 119, 15232 CAS.
- L. Grigorian, G. U. Sumanasekera, A. L. Loper, S. Fang, J. L. Allen and P. C. Eklund, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, 4195 CrossRef.
- J. VandeVondele, M. Krack, F. Mohamed, M. Parrinello, T. Chassaing and J. Hutter, Comput. Phys. Commun., 2005, 167, 103 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- S. Goedecker, M. Teter and J. Hutter, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 1703 CrossRef CAS.
- G. Henkelman, B. P. Uberuaga and H. Jonsson, J. Chem. Phys., 2000, 113(22), 9901 CrossRef CAS.
- L. U. O. Ji, W. U. Jinlei and G. Physics, Sci. China, Ser. G: Phys., Mech. Astron., 2004, 47, 685 CrossRef.
- See ESI† material at [URL *******] containing: (i) bond-lengths for all three adsorption states, (ii) local density of states for zigzag charged and neutral N-SWNTs, and (iii) local density of states for armchair charged and neutral N-SWNTs.
- G. Henkelman, A. Arnaldsson and H. Jonsson, Comput. Mater. Sci., 2006, 36, 254 CrossRef.
- E. Sanville, S. D. Kenny, R. Smith and G. Henkelman, J. Comput. Chem., 2007, 28, 899 CrossRef CAS PubMed.
- W. Tang, E. Sanville and G. Henkelman, J. Phys.: Condens. Matter, 2009, 21, 08420 Search PubMed.
- S. Yang, G.-L. Zhao and E. Khosravi, J. Phys. Chem. C, 2010, 114, 3371 CAS.
- F. Gao, G.-L. Zhao and S. Yang, ACS Catal., 2014, 4, 1267–1273 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra14023h |
| ‡ Present address: Department of Physical Science, Indian Institute of Science Education and Research Mohali, Mohali, Punjab 140306, India. |
| This journal is © The Royal Society of Chemistry 2016 |




