Synthesis of single-crystalline, porous TaON microspheres toward visible-light photocatalytic conversion of CO2 into liquid hydrocarbon fuels†
Qiutong Hanabcd,
Yong Zhou*abcd,
Lanqin Tangabcde,
Ping Liabcd,
Wenguang Tuabcd,
Liang Liabcd,
Haijin Liabcd and
Zhigang Zou*abc
aJiangsu Key Laboratory for Nano Technology, Nanjing University, Nanjing 210093, P. R. China. E-mail: zhouyong1999@nju.edu.cn
bKey Laboratory of Modern Acoustics, MOE, Institute of Acoustics, Department of Physics, Nanjing University, Nanjing 210093, P. R. China. E-mail: zgzou@nju.edu.cn
cNational Laboratory of Solid State Microstructures, Department of Physics, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing 210093, P. R. China
dEco-materials and Renewable Energy Research Center (ERERC), Nanjing University, Nanjing 210093, P. R. China
eCollege of Chemistry and Chemical Engineering, Yangcheng Institute of Technology, Yancheng 22401, P. R. China
First published on 13th September 2016
Abstract
Single-crystalline, porous TaON microspheres were prepared for photocatalysis via facile nitridation of uniform amorphous Ta2O5 sphere formed by hydrothermal treatment. The amorphous property facilitates shrinkage of Ta2O5 into the porous TaON nanostructure during the ammonification process. The porous spherical architecture of TaON plays a significant role in deciding the CO2 photocatalytic conversion efficiency into ethanal and ethanol under visible light irradiation, relative to its counterpart from commercial Ta2O5, including availability of more reaction sites, easy trapping of incident illumination, and shortening of charge transfer distance from the interior to the outer surface to expedite charge separation. Loading Pt as an electron sinker over the porous TaON, boosting the separation of the photogenerated electron–hole pairs, not only improves the photoconversion efficiency, but also alters the product species.
Introduction
The development of photocatalysts for the reduction of CO2 to hydrocarbon fuels via artificial photosynthesis is a very important means to solve energy storage and global warming problems.1–3 Artificial photosynthesis is based on the photoexcitation of semiconductors such as TiO2,4 WO3,5 CdS,6 Zn2GeO4,7 Fe2V4O13,8 and so on. A series of hydrocarbon products (e.g., C1, C2, and C3 compounds) could be obtained via different photocatalysts, which depends on the number of electrons and protons (e−/H+) involved in multiple reactions.9 Most of the currently developed photocatalysts can only show photochemical activity under ultraviolet (UV) light with the energy accounting for less than 5% of the total solar spectrum owing to the wide band gaps.10 Therefore, how to narrow the band gap of semiconductor nanostructures and enhance the utilization of visible light is of technical and commercial interest. In strict looking for a suitable catalyst for maximum use of solar energy, certain nitrogen oxide materials such as (oxy)nitrides have attracted considerable attention due to their proper band gap and the right position. Tantalum oxynitride (TaON) is one of the most towardly oxynitrides that displays remarkable advantages for the rational range of visible light absorption (λ > 420 nm). The valence band top of TaON consists of hybridization of N 2p and O 2p that is clearly different from Ta2O5 which is made up of total O 2p. According to the semiconductor energy band theory, the nitrogen 2p orbital is more potential negative compared to the oxygen 2p orbital and result in a red-shift to the visible light range.11 Photoreduction of H+ into H2 and photooxidation of H2O into O2 were early tested to assess the photocatalytic capabilities of TaON. Fabrication of an efficient TaON photoanode with cocatalyst is the first demonstration of oxynitride photoanode for photoelectrochemical water splitting into a considerable production of O2.12 TaON-based photocatalysts for oxygen evolution in a two-step water splitting system has also been successfully exhibited particularly high photocatalytic activity.13,14 Furthermore, several noble metals (Pt, Ag and Ru) were found to be potential H2-evolution O2-evolution promoters.13,15,16 While arbitrary TaON shows reasonable property in splitting water into H2 and O2, and degrading various organic contaminations,17 morphology-controlled nanostructures including hollow urchin-like sphere18 and hollow sphere19 allow further enhancement of the photocatalytic performance.In this paper, single-crystalline, porous TaON microspheres were successfully synthesized via high-temperature nitridation of uniform amorphous Ta2O5 sphere formed in isopropyl alcohol solvent. The participation of nitrogen narrows the bandgap, which availably enhances the sensitivity to visible light. As the porous spherical architecture could easily capture incident illumination and shorten of charge transfer distance from interior to outer surface to expedite charge separation, the TaON microsphere is confirmed to significantly improve the photocatalytic efficiency toward conversion of CO2 into ethanol and ethanal, under visible light irradiation, superior to its counterpart from commercial Ta2O5. By loading Pt as an electron sinker to boost the separations of the photogenerated electron–hole pairs, the photoconversion efficiency was further increased, and the product species can be altered.
Experimental section
Preparation of porous TaON microspheres
Total chemical reagents from commercial sources were of analytical grade and were not purified during use. In a emblematic synthesis, 30 ml of isopropyl alcohol solution (99.8%; Kanto Chemicals) containing the 0.1 g of TaCl5 (99.9%; High Purity Chemicals) and additionally 0.1 g of tetrabutylammonium hydroxide (10% in Water) were stirred for 30 min. A 50 ml inner volume of Teflon-lined stainless steel autoclave was prepared to take in the resulting solution. Then the synthesis was put in an electric oven to continuously heat at 200 °C for 24 h before starting to cool down naturally to room temperature. The bottom sediment was washed with deionized water and centrifugated, and dried in the freeze dryer. The white spherical structure of Ta2O5 was obtained. A suitable amount of Ta2O5 powder was then nitrogenized in a quartz tube at 1123 K for 10 h in ammonia atmosphere with flowing rate of 100 ml min−1. From the start heating to down to ambient temperature, the ammonia gas always kept flowing to avoid being oxidized gain. Finally, the yellow-green porous TaON microspheres were obtained. Meanwhile, commercial Ta2O5 powder as references is named C–Ta2O5. C–TaON was synthesized with the same method described above to nitride the commercial Ta2O5.Pt-loaded TaON was prepared by a precipitation method. 20 ml of methanol solution containing the 0.1 g of TaON powder and additionally 0.05 ml of H2PtCl6 (0.5 wt%) aqueous solution were poured into a glass beaker with 80 ml deionized water. The mixture was stirred for 5 h under the light irradiation of a 300 W Xe lamp before being washed and dried.
Characterization of porous TaON microspheres
The crystallographic phases and purity situation of the prepared powder samples were researched by X-ray diffractometry (XRD; Rigaku Ultima III, Japan) at room temperature. The XRD curve was described with a sweep rate of 10° min−1 using Cu-Kα radiation (λ = 0.154178 nm) at 40 kV and 40 mA. The morphology and core structure of samples were characterized with the field emission scanning electron microscopy (FE-SEM) on an XL30 ESEM FEG scanning electron microscopy conducting at 20 kV. The transmission electron microscopy (TEM) and fine resolution transmission electron microscope (HRTEM) images were taken on a JEM 200CX TEM apparatus. The diffuse reflectance graph of the powder was depicted with a UV-vis spectrophotometer (UV-2550, Shimadzu) and switched to the absorption spectrum on the basis of the Kubelka−Munk connection. The size of specific surface area for the sample was detected by nitrogen sorption on a surface area at 77 K and porosity analyzer (Micromeritics TriStar 3000, USA) and counted by the Brunauer–Emmett–Teller (BET) method. The adsorbing capacity of CO2 was assessed at 273 K on an automatic volumetric adsorption equipment (Micromertics ASAP 2010). The chemical state was researched by X-ray photoelectron spectroscopy (XPS; K-Alpha, Thermo Fisher Scientific).Measurement of photocatalytic activity
Photocatalytic reactions for carbon dioxide reduction were carried out in the Pyrex reaction vessel has a top irradiation area of 50.27 cm2 at room temperature. Typically, 0.1 g of the as-prepared catalyst powder was dispersed in 100 ml NaHCO3 water solution (1 mol l−1) under magnetic stirring. The high purity of CO2 gas was continuously bubbled into mixed solution for 30 minutes in order to eliminate air and provide the necessary reaction gas. The samples were irradiated by a solar simulator (Microsolar 300 xenon lamp, nominal power 50 W, diameter 2.5 cm, intensity 1.02 kW m−2) fitted with a filter to select only light at visible wavelengths (λ > 420 nm). The temperature of the reaction solution was invariably kept at room temperature by flowing of cooling water during the reaction process. Starting from the Xe lamp was lighted, about 2 ml of the reactant solution was constantly taken from the reaction vessel every hour and injected into a vial for succeeding reaction products analysis by using a liquid chromatograph (LC-2014, Agilent Technologies, USA). The irradiation time is 5 hours.Results and discussion
The color of the samples obviously change from white for as-obtained Ta2O5 to yellow green for the TaON (Fig. 1), illustrating that the oxygen atoms gradually replaced with the nitrogen atoms over increasing the nitriding time. The FE-SEM images show that the Ta2O5 of 400–500 nm in diameter displays relatively uniform, monodisperse, spherical microstructure with smooth surface (Fig. 2a1), in contrast with irregular C–Ta2O5 (See ESI†). With high-temperature nitridation, the formed TaON microspheres exhibit surface roughness and contain abundant pores (Fig. 2b2). | ||
| Fig. 2 Low and high magnification SEM images of (a1) and (a2) Ta2O5 microspheres, (b1) and (b2) single-crystalline porous TaON microspheres. | ||
The TEM images further reveal that the Ta2O5 microsphere consists of numerous extremely tiny particles (Fig. 3a1 and a2). The amorphous property of those particles (see below XRD analysis) facilitates shrinkage of Ta2O5 into the porous TaON nanostructure during the nitridation process at high temperature. The light contrast of the TaON microsphere indicates the formation of nanopore (Fig. 3b1). The corresponding diffraction dot diagram of the selected-area electron diffraction (SAED) manifests the single-crystal property of TaON microsphere. The interplanar distance between the well-defined lattice fringes is 0.42 nm, assigned to the (110) crystal plane of the TaON crystal (Fig. 3b2).
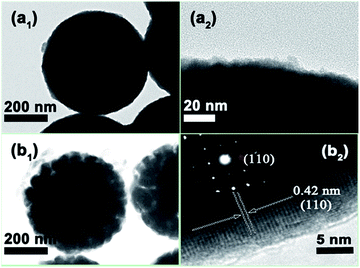 | ||
| Fig. 3 (a1) and (a2) TEM images of Ta2O5 microsphere; (b1) and (b2) TEM image of porous TaON microsphere with the inset corresponding to the SAED. | ||
The phase transformation process of samples were characterized by X-ray diffraction XRD patterns. Compared with the XRD peaks of C–Ta2O5 (Fig. 4a), the large valley packages at about 26° and 55° indicates the amorphous or poor crystallinity of the Ta2O5 microsphere (Fig. 4b). After nitridation with heating treatment, the strong diffraction peaks can well index to TaON according to JCPDS card no. 71-0178 (Fig. 4c). It was found that the nitridation time greatly affects the crystal formation of the nitride samples. Extension of the nitridation time to 15 hours led to the formation of Ta3N5 (JCPDS no. 79-1533) (Fig. 4d).
 | ||
| Fig. 4 XRD pattems of (a) C–Ta2O5; (b) Ta2O5 microsphere; (c) porous TaON microsphere; (d) Ta3N5 microsphere, and (e) standard JCPDS card (no. 71-0178) for TaON. | ||
UV-visible absorption edge of the Ta2O5 is approximately 300 nm so that it only shows the response ability in the ultraviolet (UV) light (Fig. 5). The TaON sphere displays obviously strong absorption in visible light because the nitrogen 2p orbital is more potential negative than the oxygen 2p orbital, which is advantageous to the spectral position of the band edge red-shift to around 520 nm (Fig. 5a). The band gap of the Ta2O5 and TaON microsphere were calculated to be 3.9 and 2.4 eV (Fig. 5b), respectively, according to the eqn (1):20,21
| (αhν)n = A(hν − Eg) | (1) |
 | ||
| Fig. 5 (a) UV-visible absorption spectrum of Ta2O5 microsphere and porous TaON microsphere. The band edge of (b) Ta2O5 microsphere and porous TaON microsphere. | ||
The isotherms adsorption–desorption behavior of porous TaON microsphere was detected by the BET measurement. As the pressure increases, the porous TaON microsphere received the relatively higher adsorbed volume of the nitrogen (Fig. 6). A hysteresis loop of nitrogen adsorption–desorption isotherms represents the characteristic of porous structure and adsorption without limits at high relative pressure.22 The inset of pore diameter distribution curve further reveals the average pore size diameter of about 32 nm for the porous TaON sphere. The specific surface area of porous TaON microsphere is about 11.12 m2 g−1, 1.5-time larger than the counterpart from C–Ta2O5 (7.41 m2 g−1).
 | ||
| Fig. 6 Nitrogen adsorption–desorption isotherms of porous TaON microsphere. Inset shows the corresponding pore diameter distribution. | ||
Fig. 7a displays the presence of Ta, O, and N in the full-scale XPS spectrum of TaON microsphere. The strong peaks of binding energies at 25.6 eV and 27.5 eV correspond to the spin orbit separation of the Ta 4 f5/2 and Ta 4 f7/2 ingredients, respectively (Fig. 7b), indicating the formation of the Ta5+.23 The two different binding energies of O element can be assigned to the features of Ta–O bond (530.2 eV) and oxygen in carbonate species or hydroxyl groups (531.4 eV) (Fig. 7c).20 The strong peaks for Ta 4 P2/3 and N 1s in Fig. 7d reveals that the N 1s region concentrated at 396.3 eV linked to the binding energy of approximately 403.5 eV for Ta 4 P2/3, further confirming the formation of numerous Ta–N bonds.23 Based on the bandgap of the TaON of 2.40 eV, the corresponding EVB and ECB were detected located at about 2.0 eV (Fig. 7e) and −0.4 eV, respectively, which satisfies the qualification for CO2 photoreduction potential.
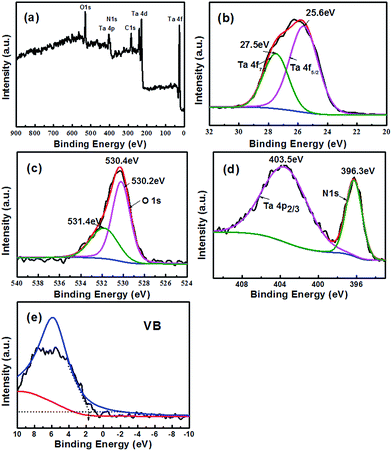 | ||
| Fig. 7 XPS spectra analysis of the TaON microsphere: (a) wide scan for sample; narrow scan for (b) Ta element, (c) O element and (d) Ta and N element; (e) location of the VB. | ||
The photocatalytic CO2 conversion was carried out with visible light (λ > 420 nm) under CO2-saturaed NaHCO3 electrolytes. The identical CO2 reduction experiment carried out in the dark or in the presence of highly pure nitrogen/argon exhibits no appearance of the hydrocarbons, indicating that the formation of ethanal and ethanol originates from input CO2 gas rather than the decomposition of any constituent. Fig. 8 shows that CO2 can be photoreduced to C2H5OH and CH3CHO by using TaON samples as photocatalysts with noble-metal co-catalysts such as Pt. The C2H5OH rate of generation of TaON and C–TaON are measured to be 2.03 μmol h−1 g−1 and 0.84 μmol h−1 g−1, respectively. Also, the TaON (CH3CHO: 0.52 μmol h−1 g−1) shows about a 3.25 times improvement in conversion rate than the referred C–TaON (0.16 μmol h−1 g−1) (Fig. 8a), which is ascribed to following reasons: (1) the mesoporous structure enhances gas capture/adsorption of the reactants and provides more reaction sites; (2) the porous spheriform structure potentially takes on a photon capture trap well to allow the multi-scattering of incidence light for enhancement of light absorption, as demonstrated in the precedent TiO2 hierarchical nanostructure;24–26 (3) the porous structure shortens charge transfer distance from interior to outer surface for expediting separation of photogenerated charge. The output in the C2H5OH of the porous TaON increased with the photocatalytic time, and a ultimate average output of C2H5OH received in the experiment after 5 h of continuous irradiation is about 11.70 μmol h−1 g−1, which is far better than a final yield of the C–TaON (4.21 μmol h−1 g−1) (Fig. 8b). The generation rate of C2H5OH over the porous TaON microspheres could be further increased to 2.34 μmol h−1 g−1 through loading Pt (0.5 wt%) as an electron sinker to boost the separations of the photogenerated electron–hole pairs.27 Interestingly, the CH3CHO generation rate was oppositely decreased after loading Pt. It is possible that Pt nanoparticles accelerate the electron transfer to allow the part of the forming CH3CHO to further continuously reduce into C2H5OH.
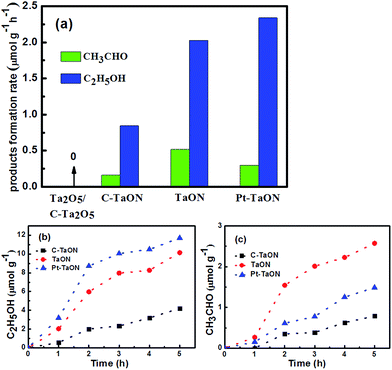 | ||
| Fig. 8 (a) Comparation of the average product formation rates of samples. Photocatalytic (b) C2H5OH and (c) CH3CHO evolution amounts for C–TaON, TaON microspheres and Pt-loaded TaON microspheres. | ||
The solar-to-chemical energy conversion efficiency over the present system was calculated using the following formula. Here the energy stored in the products is acquired from the heat energy released from complete combustion of per mole pure substance, ∇HC2H5OH = 1366KJ mol−1, ∇HC2H4O = 1168KJ mol−1 [The principle of general chemistry (fourth edition), 85], multiplied by the amount of products (see the detail in Experimental section).
 | (2) |
The average conversion rate of the solar-to-chemical energy was listed in Table 1. The irradiation energy is the solar intensity multiplied by the irradiation area and irradiation time.
| Samples | Solar-to-CH3CHO E (%) | Solar-to- C2H5OH E (%) | Total solar to hydrocarbon E (%) |
|---|---|---|---|
| C–TaON | 0.001 | 0.006 | 0.007 |
| TaON | 0.003 | 0.015 | 0.018 |
| Pt–TaON | 0.002 | 0.017 | 0.019 |
Conclusion
The single-crystalline, porous spherical architecture of TaON was successfully obtained through nitridation of the Ta2O5 microsphere precursor. This nanostructure exhibit high photocatalytic activity for reduction of CO2 to liquid hydrocarbon fuels in the presence of NaHCO3 electrolytes under visible light (λ > 420 nm) illumination, relatively to C–TaON. The porous structure allows for effectively capturing incident illumination and providing more reaction sites. Loading of Pt cocatalyst enables to further improve the photocatalytic efficiency and tune the conversion products.Acknowledgements
This work was supported by 973 Programs (No. 2014CB239302 and 2013CB632404), National Natural Science Foundation of China (No. 21473091, 51272101, and 21673001), and National Science Foundation of Jiangsu Province (No. BK20130425), Anhui Provincial Natural Science Foundation (No. 1608085ME102).Notes and references
- T. Inoue, A. Fujishima, S. Konishi and K. Honda, Nature, 1979, 277, 637 CrossRef CAS.
- P. G. Jessop, T. Ikariya and R. Noyori, Chem. Rev., 1995, 95, 259 CrossRef CAS.
- X. J. Zhang, F. Han, B. Shi, S. Farsinezhad, G. P. Dechaine and K. Shankar, Angew. Chem., Int. Ed., 2012, 51, 12732 CrossRef CAS PubMed.
- K. Maeda and K. Domen, J. Phys. Chem. Lett., 2010, 1, 2655 CrossRef CAS.
- X. Y. Chen, Y. Zhou, Q. Liu, Z. D. Li, J. G. Liu and Z. G. Zou, ACS Appl. Mater. Interfaces, 2012, 4, 3372 CAS.
- X. Zou, P. P. Wang, C. Li, J. Zhao, D. Wang, T. Asefa and G. D. Li, J. Mater. Chem., 2014, 2, 4682 RSC.
- Q. Liu, Y. Zhou, J. H. Kou, X. Y. Chen, Z. P. Tian, J. Gao, S. C. Yan and Z. G. Zou, J. Am. Chem. Soc., 2010, 132, 14385 CrossRef CAS PubMed.
- P. Li, Y. Zhou, W. G. Tu, Q. Liu, S. C. Yan and Z. G. Zou, ChemPlusChem, 2013, 78, 274 CrossRef CAS.
- W. G. Tu, Y. Zhou, H. J. Li, P. Li and Z. G. Zou, Nanoscale, 2015, 7, 14232 RSC.
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253 RSC.
- W. Chen, M. C. Chu, L. Gao, L. Q. Mao, J. Yuan and W. F. Shangguan, Appl. Surf. Sci., 2015, 324, 432 CrossRef CAS.
- R. Abe, M. Higashi and K. Domen, J. Am. Chem. Soc., 2010, 132, 11828 CrossRef CAS PubMed.
- K. Maeda, R. Abe and K. Domen, J. Phys. Chem. C, 2011, 115, 3057 CAS.
- S. S. Chen, Y. Qi, T. Hisatomi, Q. Ding, T. Asai and C. Li, Angew. Chem., Int. Ed., 2015, 54, 8498 CrossRef CAS PubMed.
- R. Abe, M. Higashi and K. Domen, ChemSusChem, 2011, 4, 228 CrossRef CAS PubMed.
- A. Nakada, T. Nakashima, K. Sekizawa, K. Maeda and O. Ishitani, Chem. Sci., 2016, 7, 4364 RSC.
- X. J. Sun, F. Wang, J. Z. Pan, G. Bai and N. Liu, Mater. Sci. Technol., 2007, 06, 1005 Search PubMed.
- Z. Wang, J. G. Hou, C. Yang, S. Q. Jiao, K. Huang and H. M. Zhu, Energy Environ. Sci., 2013, 6, 2134 CAS.
- Z. Wang, J. G. Hou, C. Yang, S. Q. Jiao, K. Huang and H. M. Zhu, J. Mater. Chem., 2012, 22, 21972 RSC.
- X. Q. Ma, Y. Chen, H. Li, X. l. Cui and Y. H. Lin, Mater. Res. Bull., 2015, 66, 51 CrossRef CAS.
- J. Z. Su, X. J. Feng, J. D. Sloppy, L. J. Guo and C. A. Grimes, Nano Lett., 2011, 11, 203 CrossRef CAS PubMed.
- Z. D. Li, Y. Zhou, G. G. Xue, T. Yu, J. G. Liu and Z. G. Zou, J. Mater. Chem., 2012, 22, 14341 RSC.
- Y. Chen, S. J. Liang, L. R. Wen, W. M. Wu, R. S. Yuan, X. C. Wang and L. Wu, Phys. Chem. Chem. Phys., 2013, 15, 12742 RSC.
- T. Y. Zhao, Z. Y. Liu, K. Nakata, S. Nishimoto, T. Murakami and A. Fujishima, J. Mater. Chem., 2010, 20, 5095 RSC.
- B. Z. Fang, Y. L. Xing, A. Bonakdarpour, S. C. Zhang and D. P. Wilkinson, ACS Sustainable Chem. Eng., 2015, 3, 2381 CrossRef CAS.
- B. Z. Fang, A. Bonakdarpour, K. Reilly, Y. L. Xing, F. Taghipour and D. P. Wilkinson, ACS Appl. Mater. Interfaces, 2014, 6, 15488 CAS.
- G. C. Xi, J. H. Ye, Q. Ma, N. Su, H. Bai and C. Wang, J. Am. Chem. Soc., 2012, 134, 6508 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra19368d |
| This journal is © The Royal Society of Chemistry 2016 |

