Functionalization of PVA to synthesize p-vinyl benzene sulfonate terpolymers – a comparative study of anticorrosion, adsorption and activation properties of the terpolymers on mild steel in 1 M HCl
R. Geethanjali* and
S. Subhashini
Department of Chemistry, Avinashilingam Institute for Home Science and Higher Education for Women, Coimbatore-641043, India. E-mail: anjalirajj@gmail.com; subhash.sethu@gmail.com
First published on 17th October 2016
Abstract
Two water soluble terpolymers viz. PVA-AAm-PVBS (polyvinyl alcohol-g-poly(acrylamide-p-vinyl benzene sulfonate)) and PVA-AA-PVBS (polyvinyl alcohol-g-poly(acrylic acid-p-vinyl benzene sulfonate)) were synthesized and characterized by FTIR, NMR, TGA and DSC. The corrosion protection efficiency of the terpolymers for mild steel in 1 M HCl has been studied by weight loss technique at various temperatures. Adsorption parameters were derived by fitting the results to Temkin isotherm. The adsorption of the inhibitors on metal surface was further confirmed by surface analysis using SEM and AFM. Kinetic parameters were also derived to understand the corrosion process. Results consistently showed that, both terpolymers are good inhibitors.
1. Introduction
Corrosion is a fundamental factor that affects economics and safety, particularly for metals and alloys. Mild steel (MS) is a type of steel alloy that contains carbon (0.2% to 2.1%) as one of the major constituents and is used in construction, car manufacturing industries, and manufacturing of bullets, knives, armours, bolt & nut, hinges, chains, pipes and so on. The corrosion of steel is inevitable when it comes in contact with the acid solutions during acid pickling, chemical cleaning and processing, ore production and oil well acidification.1,2 The use of inhibitor is an important method of protecting materials against deterioration due to corrosion, especially in acidic media.1 The applicability of polymeric compounds as corrosion inhibitors for metals in acid is a recognized area of research3–5 due to their desirable properties. The existing data in the literature about the corrosion inhibitors show that inhibitors act by adsorption through various hetero atoms. Polymers being larger moieties contain several repeating units with same or different hetero atoms, which could aid an efficient adsorption. These hetero atoms have the tendency to form a stronger coordination bond which in turn increases the inhibition efficiency.6 Synthetic polymers like polyacrylamide, polyacrylic acid, polyvinyl alcohol, polyethylene glycol, polyvinyl pyrrolidone etc., and natural polymers like, pectin, carboxymethyl cellulose, hydroxyl ethyl cellulose, gum arabic, amino acids etc., have been reported as prominent corrosion inhibitors in the recent years. To tailor polymers with some special properties, copolymers, terpolymers, quadripolymers, grafted polymers and composites were synthesized and their corrosion protecting ability was discussed in literature.5In this context, attempts were taken to design polymers such that they contain O, N and S hetero atoms which would be embedded in a water soluble matrix. The water soluble matrix should be economical since it forms the major part of the polymer. Hence the advantage of hetero atoms can be utilized without compensating economical and environmental viability. The present work emphasizes the use of two such terpolymers viz. PVA-AAm-PVBS (polyvinyl alcohol-g-poly(acrylamide-p-vinyl benzene sulfonate)) and PVA-AA-PVBS (polyvinyl alcohol-g-poly(acrylic acid-p-vinyl benzene sulfonate)) in corrosion inhibition. The synthesized polymers were characterized by FTIR, NMR, TGA and DSC. The polymers were also tested for their corrosion inhibition efficiency on mild steel using weight loss and the experimental data is fitted with several adsorption isotherms to gain information about the mode of adsorption of the inhibitor. Both thermodynamic parameters (standard adsorption enthalpy ΔHo, standard adsorption free energy ΔGo and standard adsorption entropy ΔSo) and kinetic parameters (apparent activation energy Ea and pre-exponential factor A) were calculated and discussed in detail. The surface analysis of the metal was conducted using scanning electron microscope (SEM) and atomic force microscope (AFM).
2. Experimental
2.1 Inhibitor synthesis
The terpolymer PVA-AAm-PVBS and PVA-AA-PVBS were synthesized by free radical polymerization of polyvinyl alcohol (PVA) and monomers acrylamide (AAm)/acrylic acid (AA) and p-vinyl benzene sulfonic acid sodium salt (PVBS) in distilled water. PVA (2.5 g; mol wt 140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), AAm/AA (1 g), and PVBS (0.5 g) were dissolved in 80 mL of water. The whole reaction mixture was purged with nitrogen gas for half-an-hour. 10 mL of sodium dodecyl sulfonate solution (0.03 g) were mixed into the reaction solution. The redox initiator pair, potassium persulphate-0.01 M of tetra ethyl methyl ethylene diamine (TEMED) was added slowly to the reaction mixture in order to initiate the polymerization reaction. The reaction was continued for 3 hours. The resulting white viscous solution was added to five-fold volume of acetone and the final product was precipitated. Few drops of ammonia were added to acetone to adjust the pH during the precipitation of PVA-AA-PVBS product. The precipitated product was a white rubbery mass that was washed several times with acetone–water mixture to get rid of the unreacted monomers, initiator and homopolymers. The product was dried under vacuum for 24 hours and utilized for further studies. Total yield: PVA-AAm-PVBS: 94.7%; PVA-AA-PVBS: 83.5%.
000), AAm/AA (1 g), and PVBS (0.5 g) were dissolved in 80 mL of water. The whole reaction mixture was purged with nitrogen gas for half-an-hour. 10 mL of sodium dodecyl sulfonate solution (0.03 g) were mixed into the reaction solution. The redox initiator pair, potassium persulphate-0.01 M of tetra ethyl methyl ethylene diamine (TEMED) was added slowly to the reaction mixture in order to initiate the polymerization reaction. The reaction was continued for 3 hours. The resulting white viscous solution was added to five-fold volume of acetone and the final product was precipitated. Few drops of ammonia were added to acetone to adjust the pH during the precipitation of PVA-AA-PVBS product. The precipitated product was a white rubbery mass that was washed several times with acetone–water mixture to get rid of the unreacted monomers, initiator and homopolymers. The product was dried under vacuum for 24 hours and utilized for further studies. Total yield: PVA-AAm-PVBS: 94.7%; PVA-AA-PVBS: 83.5%.
2.2 Characterization of the polymer product
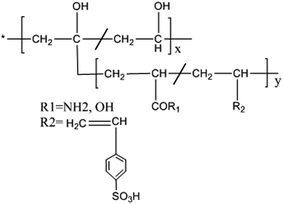
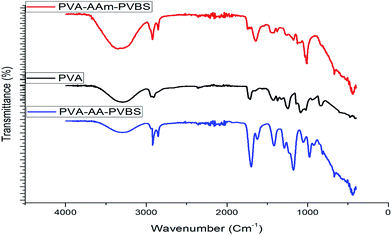
The functional groups of the grafted terpolymer were identified by IR spectroscopy (FTIR) using a Bruker Tensor-27 Fourier Transform spectrometer. Investigations have been performed in the transmission mode, using ATR assembly, at the resolution of 4 cm−1 in the range of 400–4000 cm−1. The grafted terpolymers were characterized by proton nuclear magnetic resonance (1H NMR) spectra with a DMX-500 (Bruker, Germany) and the solvent was deuterated dimethyl sulphoxide. The thermal stability of the polymers was determined using thermogravimetric analyser with an Exstar SII TG/DTA 6300 at a heating rate of 20 °C per minute from 30 to 650 °C under nitrogen purge. Differential scanning calorimetry (DSC) analysis was carried out using SDTQ 600 V8.0 Build 95 Instrument. The DSC thermograms were obtained at a heating rate of 10 °C per minute in the temperature range of 30 to 500 °C. The tentative chemical structures of the synthesized terpolymers are shown in Fig. 1.2.3 Weight loss measurements
Corrosion inhibition tests were performed with mild steel having following composition (wt%): C-0.106, Mn-0.196, P-0.027, Cr-0.022, S-0.016, Ni-0.012, Si-0.006, Mo-0.003, and remainder of Fe. The aggressive solutions of 1.0 M HCl were prepared by dilution of analytical grade 37% HCl with distilled water.Weight-loss (mg cm−2) was determined in an open system at various temperatures in the presence and absence of the inhibitor following the ASTM procedure. The strips were immersed in triplicates in 1 M HCl in the absence and presence of various concentrations (0.03, 0.06, 0.18, 0.27, 0.36, 0.45 wt%) of the inhibitor at 303–343 K. The MS sheets of 5 cm × 1 cm × 2 mm were abraded by emery paper and then washed with distilled water and acetone. After immersing for the specified time, the specimens were taken out, washed under running water in order to remove the corrosion product, dried and re-weighed accurately.
The corrosion rate (CR) and inhibitor efficiency (IE) were calculated from the following equations:
 | (1) |
 | (2) |
In the attempt to fit the θ values to various isotherms, Temkin isotherm provided a best fit. The general form of Temkin isotherm is given as:
 | (3) |
The free energy of adsorption ΔGads is calculated using the relationship:7
 | (4) |
Thermodynamic parameters such as entropy of adsorption ΔSads and enthalpy of adsorption ΔHads were deduced from the integrated form of Van't Hoff equation.8
 | (5) |
In the plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kads versus 1/T, ΔSads and ΔHads can be calculated from the slope (−ΔHads/R) and intercept (ΔSads/R + ln
Kads versus 1/T, ΔSads and ΔHads can be calculated from the slope (−ΔHads/R) and intercept (ΔSads/R + ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/55.5) respectively.
1/55.5) respectively.
The temperature dependency of the corrosion reaction can be evaluated through the activation parameters (Ea, ΔHo and ΔSo) using Arrhenius equation and transition state eqn (6) and (7).9
 | (6) |
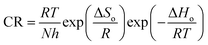 | (7) |
2.4 Surface analysis of the metal samples
The surface examination of the metal samples was carried out using scanning electron microscope (Bruker). EDX system attached with SEM was used for chemical characterization of the film formed on steel surface. AFM analysis was carried out by immersing the mild steel strips in inhibited (0.45 wt% inhibitor) and uninhibited solution for 6 hours, cleaning the specimens in distilled water, drying and imaging using AFM instrument (A.P.E research, model A100, Italy).3. Results and discussion
3.1 Characterization
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str. of acrylic acid unit. The C–O–H/C–H bending vibrations are figured at 1438 cm−1 and 1456 cm−1 for PVA-AAm-PVBS and PVA-AA-PVBS terpolymer respectively. Orler et al.10 observed peaks at 1128, 1175 and 1095 cm−1 for styrene sulphonic acid. In the case of PVA-AAm-PVBS, the sulfonic peaks appear at 1197–1098 cm−1 for asymmetric SO3− stretching and at 1009 cm−1 for symmetric SO3− stretching. Similarly, for PVA-AA-PVBS, these sulfonic peaks appear around 1180 cm−1 for asymmetric SO3− stretching and at 1050 cm−1 for symmetric SO3− stretching. The out-of-plane bending vibrations of Ar-H bond of the substituted benzene ring are manifested from the peaks at 660 and 446 cm−1 in the PVA-AAm-PVBS and at 678 and 455 cm−1 in PVA-AA-PVBS.
O str. of acrylic acid unit. The C–O–H/C–H bending vibrations are figured at 1438 cm−1 and 1456 cm−1 for PVA-AAm-PVBS and PVA-AA-PVBS terpolymer respectively. Orler et al.10 observed peaks at 1128, 1175 and 1095 cm−1 for styrene sulphonic acid. In the case of PVA-AAm-PVBS, the sulfonic peaks appear at 1197–1098 cm−1 for asymmetric SO3− stretching and at 1009 cm−1 for symmetric SO3− stretching. Similarly, for PVA-AA-PVBS, these sulfonic peaks appear around 1180 cm−1 for asymmetric SO3− stretching and at 1050 cm−1 for symmetric SO3− stretching. The out-of-plane bending vibrations of Ar-H bond of the substituted benzene ring are manifested from the peaks at 660 and 446 cm−1 in the PVA-AAm-PVBS and at 678 and 455 cm−1 in PVA-AA-PVBS.
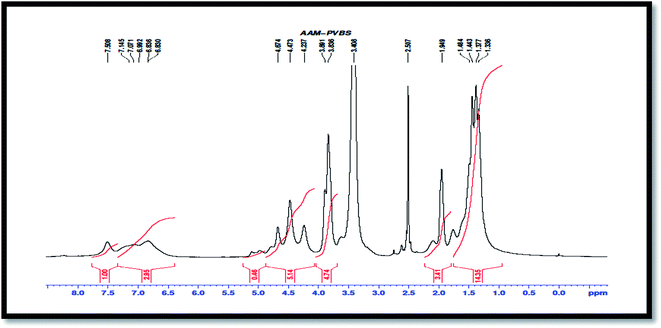
PVA-AAm-PVBS. 1H NMR (500 MHz, DMSO-d6, δ ppm): 1.33–1.5 (CH2), 1.94 (CH), 3.83–3.89 (CH–OH), 4.67, 4.47, 4.23 (t, Ar–H), 5–5.1 (CONH), 7.5 (Ha Ar-CH), 6.83 (Hb Ar-CH).
PVA-AA-PVBS. 1H NMR (500 MHz, DMSO-d6, δ ppm): 1.33–1.5 (CH2), 1.95 (CH), 3.7–3.8 (CH–OH), 7.4 and 6.7 (Ar-CH).
The weak multiple signal owing to the unsaturated pattern in the region of 7.5 ppm and 6.83 ppm in PVA-AAm-PVBS (Fig. 3), and 7.4 ppm and 6.7 ppm in PVA-AA-PVBS (Fig. 4) are due to aromatic protons11 of p-vinyl benzene sulfonic acid sodium salt. The amide proton signal is shifted to the upfield region to 5.1–5 ppm which may be due to hydrogen bonding.12 A triplet signal appeared in the region of 4.67 ppm, 4.47 ppm and 4.23 ppm corresponds to the benzyl proton of PVBS.13 The benzyl protons are not distinctively shown in the PVA-AA-PVBS because of the extensive overlapping of OH of PVA and COOH of acrylic acid in the specified region. The solvent peak of DMSO-D6 appears at 2.5 ppm, 3.4 ppm and 2.3 ppm.
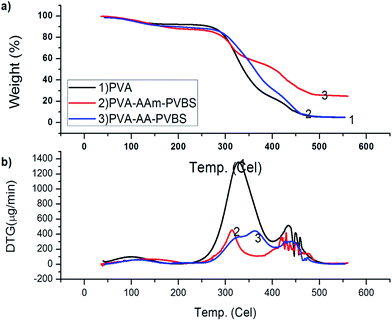
PVA-AA-PVBS undergoes a three step degradation process and is exhibited in the form of three peaks in the DTG curve (Fig. 5b). According to DTG curves, the first step is small and the main degradations occur in the second and third steps. The first step with a weight loss of 7% can be ascribed to the water loss. The second degradation step reflects a large peak with two peak maxima at 317 and 362 °C. At this stage 50% of the degradation has taken place corresponding to decarboxylation and desulfonation from the terpolymer. Similar to PVA-AAm-PVBS, the final decomposition at 416 °C resulting in 74% weight loss from the initial mass can be ascribed to the breakdown of C–H bonds, decompositions of any intermediate macromolecular product, and degradation of aromatic fragments of styrene units. The complete degradation of the terpolymer starts at 450 °C.
The DSC profile of the PVA-AAm-PVBS (Fig. 6) exhibits three different melting endotherms. The melting onset is observed at 205 °C, with first melting peak positioned at 311 °C with an enthalpy of 173 mJ mg−1 and the second melting peak positioned at 418 °C with an enthalpy of 46.2 mJ mg−1. The third melting peak is recorded at 477 °C, whereas the shape of the curve indicates the melting is accompanied with decomposition. When compared to the pure PVA, the melting point is shifted well to a higher temperature indicating the extensiveness of cross-linking of the polymeric chain which makes them harder to melt. The glass transition occurs around 100 °C with an enthalpy relaxation of 185 °C. When the Tg value of the PVA-AAm-PVBS is compared to that of pure PVA, it is shifted slightly to higher values as a result of crosslinking with other monomer reactants. The increase in the Tg value is due to the dispersion of the polyacrylamide and p-vinylbenzene sulfonic acid segments in the PVA matrix which basically hinders the motion of chains of the polymer.18
The DSC trace of PVA-AA-PVBS (Fig. 6) displays two endothermic peaks. The first peak at 189 °C can be termed as a thermal effect due to melting of some segments of PVA. A sharp endothermic melting transition at 259 °C (ΔH = 11.2 mJ mg−1) with a melting onset of 223 °C (ΔH = 46.2 mJ mg−1) is assignable to the melting of the complete polymer. The melting is followed by decomposition of the polymer at 358 °C. The glass transition of PVA-AA-PVBS is observed with decrease in heat capacity in the temperature range of 35–119 °C with midpoint of 80 °C. Tg is highly dependant on polymer chain branching. Linear polymer chains possess smaller free volume than their branched counterparts and reflect higher Tg values. But in the branched polymers, if the branches contain bulkier group they impose hindrance or restriction to segmental motion, for which higher Tg values are expected. Nevertheless, crosslinked polymers also reflect higher Tg due to their restricted segmental motion, and sometimes the cross-linking is very high such that the polymer undergoes decomposition before starting the segmental motion.19 The Tg value obtained for the PVA-AA-PVBS is consistent with the values obtained for pure PVA. Grafting of PVA with acrylic acid and p-vinyl benzene sulfonate did not alter the Tg of pure PVA, because the polymerization neither imparted additional mobility or hindered mobility of the segments of PVA.
3.2 Weight loss – effect of concentration and temperature on corrosion rate
The effect of temperature on the inhibited acid–metal reaction is very complex because of certain changes that occur: rapid etching, desorption of the inhibitor film and decomposition of inhibitor at higher temperature.20 To investigate the mechanism of inhibition and to calculate the activation energies of the corrosion process, gravimetric measurements were taken at various temperatures (303–343 K) in the absence and presence of 0.03–0.45 wt% of the PVA-AAm-PVBS and PVA-AA-PVBS for 1/2 h of immersion. The values of inhibition efficiency obtained from the weight loss at various temperatures are provided in the Table 1.| Conc. (wt%) | 303 K | 313 K | 323 K | 333 K | 343 K | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CR (mpy) | IE (%) | CR (mpy) | IE (%) | CR (mpy) | IE (%) | CR (mpy) | IE (%) | CR (mpy) | IE (%) | |
| PVA-AAm-PVBS | ||||||||||
| Blank | 870.0 | 1825.3 | 5680.6 | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 713.1 713.1 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 449.3 449.3 |
||||
| 0.03 | 366.8 | 57.8 | 776.2 | 57.5 | 1876.5 | 67.0 | 2755.0 | 74.3 | 5134.8 | 72.7 |
| 0.09 | 315.6 | 63.7 | 699.4 | 61.7 | 1398.8 | 75.4 | 2268.8 | 78.8 | 4025.9 | 78.6 |
| 0.18 | 298.5 | 65.7 | 614.1 | 66.4 | 1160.0 | 79.6 | 2013.0 | 81.2 | 3829.7 | 79.7 |
| 0.27 | 290.0 | 66.7 | 520.3 | 71.5 | 1194.1 | 79.0 | 1944.7 | 81.8 | 3616.5 | 80.8 |
| 0.36 | 290.0 | 66.7 | 511.8 | 72.0 | 1177.1 | 79.3 | 1868.0 | 82.6 | 3565.3 | 81.1 |
| 0.45 | 272.9 | 68.6 | 469.1 | 74.3 | 1125.9 | 80.2 | 1620.6 | 84.9 | 3352.1 | 82.2 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||
| PVA-AA-PVBS | ||||||||||
| Blank | 870.0 | 1825.3 | 5680.6 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 713.1 713.1 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 449.3 449.3 |
|||||
| 0.03 | 452.1 | 48.0 | 861.5 | 52.8 | 1808.3 | 68.2 | 3198.6 | 70.1 | 6567.7 | 65.1 |
| 0.09 | 366.8 | 57.8 | 733.5 | 59.8 | 1731.5 | 69.5 | 2959.7 | 72.4 | 6397.1 | 66.0 |
| 0.18 | 349.7 | 59.8 | 716.5 | 60.8 | 1791.2 | 68.5 | 2362.7 | 77.9 | 5271.2 | 72.0 |
| 0.27 | 307.1 | 64.7 | 622.7 | 65.9 | 1501.2 | 73.6 | 2268.8 | 78.8 | 4333.0 | 77.0 |
| 0.36 | 307.1 | 64.7 | 588.5 | 67.8 | 1330.6 | 76.6 | 2149.4 | 79.9 | 3966.2 | 78.9 |
| 0.45 | 264.4 | 69.6 | 520.3 | 71.5 | 1245.3 | 78.1 | 1748.5 | 83.7 | 3923.6 | 79.2 |
Observation of Table 1 shows that the corrosion rate was found to increase in the presence and absence of the inhibitor. The increase in corrosion rate was found to be more pronounced with increase in temperature for blank HCl solution. However, after the addition of inhibitors the increase in corrosion rate with rise in temperature was less pronounced. This ensures the protective ability of the polymer. In the present systems studied, the IE gradually increased in the temperature domain range of 303–333 K (68.6% to 84.9% for 0.45 wt% of inhibitor) and then decreased to 82.2% at 343 K i.e. the protective nature of the film is interfered at 343 K rendering a less protective film which in turn allows the corrodant species to diffuse into the active sites initiating the corrosion.21
Because increase in temperature stimulates kinetic energy of the metal surface which adversely affects the adsorption process. Hence the adsorption–desorption equilibrium is shifted more towards the desorption process21 and the electrode surface is more roughened owing to the enhanced corrosion. This fact can be explained on the basis of structural orientation according to Fares et al. (2012a)22 and Chamovska et al. (2007)23 as follows (Fig. 7): at lower temperatures coiled structure of the polymer chains covers the surface and prevents the metal dissolution. But at temperatures from 313–333 K, the long chains of the coiled structure should have elongated enabling better surface coverage providing the better efficiency. At temperatures greater than 333 K, some broken fragments could have started desorbing from the metal surface and consequently IE starts decreasing.
 | ||
| Fig. 7 Plausible transformations of the terpolymer at various temperatures on mild steel surface at various temperatures. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C. The linear regression between θ vs. ln
C. The linear regression between θ vs. ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C for PVA-AA-PVBS at various temperatures is depicted in Fig. 8. Thermodynamic adsorption parameters are a useful tool for clarifying the adsorption behaviour of an inhibitor. Table 2 summarises the adsorption parameters derived from Temkin isotherm.
C for PVA-AA-PVBS at various temperatures is depicted in Fig. 8. Thermodynamic adsorption parameters are a useful tool for clarifying the adsorption behaviour of an inhibitor. Table 2 summarises the adsorption parameters derived from Temkin isotherm.
 | ||
| Fig. 8 Temkin isotherm for MS in 1 M HCl in the presence of different concentrations of PVA-AA-PVBS. | ||
| Temperature | Statistical analysis | f | Kads × 105 | ΔGads (kJ mol−1) | ΔHads (kJ mol−1) | ΔSads (J mol−1 K−1) | |
|---|---|---|---|---|---|---|---|
| R2 | F | ||||||
| PVA-AAm-PVBS | |||||||
| 303 | 0.92 | 44.90[0.003] | 17.20 | 1.54 × 105 | −40.21 | 346.13 | 1258.1 |
| 313 | 0.94 | 128.68[0.000] | 16.11 | 2.88 × 105 | −43.17 | ||
| 323 | 0.92 | 415.04[0.000] | 18.26 | 6.65 × 106 | −52.98 | ||
| 333 | 0.93 | 154.10[0.000] | 28.03 | 3.89 × 1010 | −78.65 | ||
| 343 | 0.89 | 65.35[0.001] | 30.83 | 2.40 × 1011 | −86.20 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| PVA-AA-PVBS | |||||||
| 303 | 0.91 | 46.88[0.002] | 23.53 | 2.03 × 107 | −52.51 | 136.9 | 193.13 |
| 313 | 0.97 | 62.93[0.001] | 15.68 | 1.19 × 105 | −40.87 | ||
| 323 | 0.99 | 46.01[0.002] | 22.29 | 5.92 × 107 | −58.86 | ||
| 333 | 0.97 | 49.92[0.002] | 21.01 | 6.58 × 107 | −60.97 | ||
| 343 | 0.94 | 33.19[0.005] | 16.93 | 1.41 × 106 | −51.85 | ||
The efficiency of an inhibitor is pertained to the magnitude of its binding constant ‘Kads’,23 therefore larger values of Kads implies a stronger interaction of the inhibitor molecules with the metal surface. Also the increase in Kads values indicates the increase in strength of the adsorption between inhibitor molecules and metal. The increase in Kads values with temperature is directly proportional to the adsorption efficiency of the inhibitor with temperature.24 In this study, the magnitude of the Kads values are larger which is attributable to the effective adsorption of the inhibitor.25 Basically, the constant f describes the intermolecular interaction in the adsorbed layer and on the heterogeneity of the surface. If f is positive, mutual repulsion of molecules occurs and if f is negative mutual attraction of molecules takes place. The values of f retrieved from the present investigation are positive indicating repulsive forces that exist between inhibitor molecules in the adsorbed layer.
Free energy of adsorption. The negative values of ΔGads indicate the spontaneity of the adsorption process. Generally, the free energy values less than −20 kJ mol−1 are attributed to the electrostatic interaction between charged molecules and charged metal surface, and the phenomenon is termed as physisorption. The free energy values greater than −40 kJ mol−1 or more involve charge sharing or transfer from the inhibitor molecules to the metal surface to form a coordinate covalent bond, and the phenomenon is associated with chemisorption.26–28 The values in the range of 21–39 kJ mol−1 can be attributed to the threshold for chemical adsorption in combination with physical adsorption.28–31 In the present investigation the ΔGads values are negative indicating the spontaneous adsorption process, and the magnitude is greater than −40 kJ mol−1 (−40 to −86 kJ mol−1) which is consistent with the chemical mode of adsorption as a result of sharing of electrons between the inhibitor molecule and metal surface to form a co-ordinate bond. The ΔGads values increase with increasing temperature which can be assigned to the fact of strong chemical adsorption at higher temperatures. PVA-AAm-PVBS assumes a highest mean ΔGads value −60.2 kJ mol−1 indicating the highest charge transfer had taken place between the terpolymer and metal surface.
Enthalpy of adsorption and entropy of adsorption. The sign of the ΔH can be used for distinguishing the adsorption of inhibitor as exothermic process or endothermic process. The positive ΔHads reflects the endothermic process and negative values of ΔHads reflects the exothermic process. Endothermic process is generally attributed to chemisorption. But exothermic process can be associated with physisorption or chemisorption, depending on absolute values. A physisorption process reflects an enthalpy around 40 kJ mol−1 while chemisorption process results an enthalpy around 100 kJ mol−1.28,31 In the present study the enthalpy is positive and hence can be unequivocally considered as chemisorption. The orderliness/disorderliness of an adsorption process can be determined using entropy of adsorption. The positive values of ΔSads might be explained in the following way: the positive entropy is attributed to the solvent entropy that is associated with the disorderliness of the solution during the adsorption process.32
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CR vs. 1/T is the Arrhenius plot. The activation energy Ea is calculated from the slope (−Ea/R) of the straight lines and the plot of log
CR vs. 1/T is the Arrhenius plot. The activation energy Ea is calculated from the slope (−Ea/R) of the straight lines and the plot of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CR/T vs. 1/T is the transition state plot. The transition state plot is also a set of straight lines with the slope value of −ΔHo/R and intercept of (log(R/Nh) + ΔSo/R) from which the values of ΔHo and ΔSo are calculated. Fig. 9a and b shows the Arrhenius and transition state plot of PVA-AAm-PVBS. The kinetic parameters such as Ea, ΔHo and ΔSo were calculated and summarised in the Table 3.
CR/T vs. 1/T is the transition state plot. The transition state plot is also a set of straight lines with the slope value of −ΔHo/R and intercept of (log(R/Nh) + ΔSo/R) from which the values of ΔHo and ΔSo are calculated. Fig. 9a and b shows the Arrhenius and transition state plot of PVA-AAm-PVBS. The kinetic parameters such as Ea, ΔHo and ΔSo were calculated and summarised in the Table 3.
| Inhibitor | Conc. (wt%) | Ea (kJ mol−1) | ΔHo (kJ mol−1) | ΔSo (J mol−1 K−1) | λ × 1014 |
|---|---|---|---|---|---|
| 1 M HCl | 68.27 | 65.59 | 27.74 | 5.130 | |
| PVA-AAm-PVBS | 0.03 | 56.72 | 54.04 | −17.17 | 5.130 |
| 0.09 | 54.36 | 51.68 | −25.99 | 0.023 | |
| 0.18 | 54.48 | 51.80 | −26.36 | 0.008 | |
| 0.27 | 55.03 | 52.35 | −25.40 | 0.008 | |
| 0.36 | 54.55 | 51.87 | −27.09 | 0.009 | |
| 0.45 | 54.00 | 51.32 | −29.56 | 0.007 | |
| PVA-AA-PVBS | 0.03 | 61.32 | 58.64 | −2.03 | 0.143 |
| 0.09 | 62.34 | 59.66 | 0.51 | 0.194 | |
| 0.18 | 59.07 | 56.39 | −10.71 | 0.050 | |
| 0.27 | 57.04 | 54.36 | −17.82 | 0.021 | |
| 0.36 | 57.03 | 54.36 | −18.51 | 0.020 | |
| 0.45 | 57.13 | 54.45 | −19.00 | 0.019 |
The R2 values obtained for both the plots are close to unity indicating the applicability of kinetic model for the corrosion process. Inspection of the Ea values reveal that the values obtained for blank HCl is 68 kJ mol−1 which is in agreement with the literature data for iron dissolution in HCl in the range of 58–100 kJ mol−1.32–34 The Ea values obtained for inhibited solution is lesser than those obtained for blank HCl and lies in the range of 57–64 kJ mol−1. The activation energy is lesser than the uninhibited solution, and IE increases with increase in temperature till 343 K and then decreases. The aforementioned facts are tenable to the chemisorption mode of adsorption. The decreased Ea is explained by various authors in different perspectives as follows: Riggs et al.35 report that this decrease in the activation energy of corrosion at maximum inhibited level is a result of shift of the net corrosion reaction from that on the uncovered portion to covered portion of the metal surface. In other words, when the surface coverage is maximum, diffusion through the inhibitor layer or corrosion products will become the rate-determining step of the metal dissolution process.35
According to Noor and Al-Moubaraki,36 with increase in temperature, due to some chemical changes that occur in the inhibitor molecules, electron density increases at the adsorption centres of the molecule which in turn furnishes improved inhibition efficiency. In other words the decrease in apparent activation energy is a result of shift of the net corrosion reaction from uncovered surface to adsorbed sites directly. The tendency of variation of Arrhenius frequency factor λ is similar to that in apparent activation energy for most of the corrosion reactions. According to Hoar and Holliday37 this can be pertained to the slow rate of inhibitor adsorption with a resultant closer approach to equilibrium during the experiments at higher temperature. Schmid and Huang38 & Hegazy and Zaky39 explain the same phenomenon as follows: when the inhibitor molecules is capable of inhibiting both anodic and cathodic partial reactions on the electrode surface while a parallel reaction takes place on the inhibitor covered surface, the reaction rate of the covered surface is considerably less than that on the uncovered surface area.
The positive values of ΔHo reflect the endothermic metal dissolution process suggesting the slow process of metal dissolution in the presence of inhibitors. All the Ea values are larger than the analogous ΔHo values showing that the corrosion process basically involved a gaseous reaction i.e. hydrogen evolution reaction associated with decrease in total reaction volume.40 Hence the corrosion process can be considered as a unimolecular reaction which obeys the relation Ea − ΔHo = RT. The results permit to verify the relation between Ea and ΔHo: Ea − ΔHo = RT, i.e. the calculated values of RT are very close to the theoretical value 2.68 kJ mol−1.41
The positive values of ΔSo resulted from the blank HCl show an increase in disorderliness that takes place during a rigorous metal dissolution. At the same time the negative values of ΔSo obtained after the addition of inhibitor indicate that the rate determining step is association rather than the dissociation. This means that a decrease in disordering takes place on going from reactants to the activated complex.37,42,43
3.3 Surface analysis
 | ||
| Fig. 10 SEM images and EDX spectra of mild steel immersed in the presence of (a) PVA-AAm-PVBS (b) PVA-AA-PVBS. | ||
Fig. 10b shows the electronic image acquisition of the MS surface in the presence of PVA-AA-PVBS. The SEM image shows a uniform layer covering the surface with distributed spherical particles of various sizes. The image clearly shows the presence of two phases. Extensively cross-linked floral structure located in the ring shaped aggregated white particles. EDX mapping also confirmed the presence of iron, oxygen and sulphur.
In the present study, morphological analysis of the surfaces of the mild steel were carried out by AFM in the range 0 to 50 μm at room temperature after immersion in different test solutions for 6 h. Three-dimensional AFM images of mild steel, mild steel immersed in 1 M HCl and mild steel immersed in 1 M HCl containing inhibitors are shown in Fig. 11a–d respectively. The roughness parameter Ra and root mean square roughness Rms were calculated using software called Gwyddion and are tabulated in Table 4.
 | ||
| Fig. 11 Atomic force microscopic images of (a) polished MS; (b) MS immersed in 1 M HCl; (c) PVA-AAm-PVBS; (d) PVA-AA-PVBS. | ||
| Mild steel | Ra (μm) | Rms (μm) |
|---|---|---|
| Polished | 0.112 | 0.146 |
| Corroded | 0.567 | 0.693 |
| Inhibited by PVA-AAm-PVBS | 0.220 | 0.294 |
| Inhibited by PVA-AA-PVBS | 0.290 | 0.369 |
The polished mild steel is absolutely smooth with very least surface roughness. In the absence of the inhibitors (Fig. 11b), the surface of the film shows several mountain like formations that correspond to rough surface.45 The Ra and Rms corresponding to the MS corroded in blank HCl is 0.567 μm and 0.693 μm which are very higher than the roughness parameters obtained for inhibited surfaces. In the presence of inhibitors, the ridges caused by corrosive environment are decreased to a greater extent and a smooth surface is perceived. But still the surface resembles sand dunes which can be correlated to the uneven inhibitor film formed on MS surface during the course of the study.
4. Conclusions
The synthesized terpolymers are excellent inhibitors for the corrosion of mild steel in 1 M HCl, and the maximum inhibition efficiency is about 83% at 333 K. The inhibition efficiency values increase with respect to the increasing inhibitor concentration and temperature (till 333 K and then decreases). The adsorption of the terpolymers on mild steel surface follows Temkin adsorption isotherm. The nature of adsorption process is chemisorption. The chemisorption is spontaneous and endothermic process accompanied by positive entropy. The values of apparent activation energy (Ea) of the tested inhibitors are smaller than that in bare HCl solution which also confirms chemical adsorption. The surface analysis techniques (SEM and AFM) show that the inhibitors are distributed on the metal surface.Acknowledgements
One of the authors, R. Geethanjali thanks Tamil Nadu State Council for Science and Technology for catalysing and financially supporting the research work under RFRS scheme (TNSCST/RFRS/VR/2013–14).References
- G. Trabanelli, Inhibitors an old remedy for a new challenge, Corrosion, 1991, 47, 410–419 CrossRef CAS.
- D. D. N. Singh, T. B. Singh and B. Gaur, The role of metal cations in improving the inhibitive performance of hexamine on the corrosion of steel in hydrochloric acid solution, Corros. Sci., 1995, 37, 1005–1019 CrossRef CAS.
- M. N. Desai, B. C. Thakar and P. M. Chhaya, The Inhibitor Effectiveness of Polyamines and Ethanolamine for Acid Corrosion of Al, Corros. Sci., 1979, 19, 9–18 Search PubMed.
- R. R. Annand, R. M. Hurd and N. Hackerman, J. Electrochem. Soc., 1965, 144(112), 138–144 CrossRef.
- A. Ali Fathima Sabirneeza, R. Geethanjali and S. Subhashini, Polymeric Corrosion Inhibitors for Ferrous and Its Alloys – A Review, Chem. Eng. Commun., 2015, 202(2), 232–244 CrossRef.
- F. Bentiss, M. Traisnel and M. Lagrenee, A new triazole derivative as inhibitor of the acid corrosion of mild steel: electrochemical studies, weight loss determination, SEM and XPS, Appl. Surf. Sci., 1999, 152, 237–249 CrossRef CAS.
- S. A. Umoren, U. M. Eduok, A. U. Israel, I. B. Obot and M. M. Solomon, Coconut Coir Dust Extract: A Novel Eco-Friendly Corrosion Inhibitor for Al in HCl Solutions, Green Chem. Lett. Rev., 2012, 5(3), 303–313 CrossRef CAS.
- N. O. Obi-Egbedi and I. B. Obot, Inhibitive properties, thermodynamic and quantum chemical studies of all oxazine on mild steel corrosion in H2SO4, Corros. Sci., 2011, 53(1), 263–275 CrossRef CAS.
- Y. Zhu, M. L. Free and G. Yi, The effects of surfactant concentration, adsorption, aggregation, and solution conditions on steel corrosion inhibition and associated modeling in aqueous media, Corros. Sci., 2016, 102, 233–250 CrossRef CAS.
- E. B. Orler, D. J. Yontz and R. B. Moore, Sulfonation of Syndiotactic Polystyrene for Model Semicrystalline Ionomer Investigations, Macromolecules, 1993, 26, 5157 CrossRef CAS.
- J. C. Yang, M. J. Jablonsky and J. W. Mays, NMR and FT-IR studies of sulfonated styrene-based homopolymers and copolymers, Polymer, 2002, 43, 5125–5132 CrossRef CAS.
- M. K. S. Batista, L. F. Pinto, C. A. R. Gomes and P. Gomes, Novel highly-soluble peptide–chitosan polymers: chemical synthesis and spectral characterization, Carbohydr. Polym., 2006, 64, 299–305 CrossRef CAS.
- B. Hiran lal and S. N. Paliwal, Synthesis and Free Radical Copolymerization of N-[4-N-(Benzyl amino-carbonyl)phenyl]maleimide with Methyl Methacrylate, Malaysian Polymer Journal, 2008, 3(2), 1–12 Search PubMed.
- O. Hazer, C. Soykan and S. Kartal, Synthesis and Swelling Behavior Analysis of Poly(acrylamidoxime-co-2-acrylamido-2-methylpropane sulfonic acid) Hydrogels, J. Macromol. Sci., Part A: Pure Appl. Chem., 2007, 45(1), 45–51 CrossRef.
- C. Zhang and A. J. Easteal, Thermoanalytical, spectroscopic, and morphological study of poly(ethylene glycol)/poly(2-acrylamido-2-methylpropanesulfonic acid-co-N-isopropylacrylamide) semi-interpenetrating network gels, Appl. Polym. Sci., 2007, 104(3), 1723–1731 CrossRef CAS.
- S. Kavlak, A. Guner and Z. M. O. Rzayev, Functional Terpolymers Containing Vinylphosphonic Acid: The Synthesis and Characterization of Poly(vinylphosphonic acid-co-styrene-co-maleic anhydride), Appl. Polym. Sci., 2012, 125, 3617–3629 CrossRef CAS.
- W. Osiris Guirguis, T. H. Manal and H. Moselhey, Thermal and structural studies of poly(vinyl alcohol) and hydroxypropyl cellulose blends, Natural Science, 2012, 4(1), 57–67 CrossRef.
- Y. Gao, C. He, Y. Huang and F. L. Qing, Novel water and oil repellent POSS-based organic/inorganic nanomaterial: preparation, characterization and application to cotton fabrics, Polymer, 2010, 51(25), 5997–6004 CrossRef CAS.
- P. J. Sinko and S. Yashveer, Martin's Physical Pharmacy and Pharmaceutical Sciences Physical Chemical and Biopharmaceutical Principles in the Pharmaceutical Sciences, 6th edn, 2011, published as ch. 20 Search PubMed.
- S. Belkaid, K. Tebbji, A. Mansri, A. Chetouani and B. Hammouti, Poly(4-vinylpyridine-hexadecyl bromide) as corrosion inhibitor for mild steel in acid chloride solution, Res. Chem. Intermed., 2012, 38(9), 2309–2325 CrossRef CAS.
- I. O. Arukalam, I. C. Madufor, O. Ogbobe and E. Oguzie, Experimental and Theoretical Studies of Hydroxyethyl Cellulose as Inhibitor for Acid Corrosion Inhibition of Mild Steel and Aluminium, Open Corros. J., 2014, 6, 1–10 CrossRef.
- M. M. Fares, A. K. Maayta and M. M. Al-qudah, Pectin as promising green corrosion inhibitor of aluminum in hydrochloric acid solution, Corros. Sci., 2012, 60, 112–117 CrossRef CAS.
- D. Chamovska, M. Cvetkovska and T. Grchev, Corrosion inhibition of iron in hydrochloric acid by polyacrylamide, J. Serb. Chem. Soc., 2007, 72(7), 687–698 CrossRef CAS.
- N. Khalil, F. Mahgoub, B. A. Abd-El-Nabey and A. Abdel-Aziz, Corrosion of aluminium in perchloric acid in presence of various inorganic additives, Corros. Eng., Sci. Technol., 2003, 38(3), 205–210 CrossRef CAS.
- S. K. Shukla and E. E. Ebenso, Corrosion Inhibition, Adsorption Behavior and Thermodynamic Properties of Streptomycin on Mild Steel in Hydrochloric Acid Medium, Int. J. Electrochem. Sci., 2011, 6, 3277–3291 CAS.
- A. K. Singh and M. A. Quraishi, Investigation of Adsorption of Isoniazid Derivatives at Mild Steel/Hydrochloric Acid Interface: Electrochemical and Weight Loss Methods, Mater. Chem. Phys., 2010, 123(2–3), 666–777 CrossRef CAS.
- I. Ahamad and M. A. Quraishi, Bis(benzimidazol-2-yl)disulphide: an efficient water soluble inhibitor for corrosion of mild steel in acid media, Corros. Sci., 2009, 51(9), 2006–2013 CrossRef CAS.
- I. B. Obot and N. O. Obi-Egbedi, Fluconazole as an inhibitor for aluminium corrosion in 0.1 M HCl, Colloids Surf., A, 2008, 330(2–3), 207–212 CrossRef CAS.
- E. Noor and A. H. Al-Moubaraki, Thermodynamic study of metal corrosion and inhibitor adsorption processes in mild steel/1-methyl-4[4′(-X)-styryl pyridinium iodides/hydrochloric acid systems, Mater. Chem. Phys., 2008, 110(1), 145–154 CrossRef CAS.
- Y. Zhu, M. L. Free and J. H. Cho, Integrated evaluation of mixed surfactant distribution in water–oil-steel pipe environments and associated corrosion inhibition efficiency, Corros. Sci., 2016, 110, 213–227 CrossRef CAS.
- X. Li, S. Deng, H. Fu and G. Mu, Synergistic inhibition effect of rare earth cerium(IV) ion and anionic surfactant on the corrosion of cold rolled steel in H2SO4 solution, Corros. Sci., 2008, 50, 2635–2645 CrossRef CAS.
- M. I. Awad, Eco friendly corrosion inhibitors: inhibitive action of quinine for corrosion of low carbon steel in 1 M HCl, Appl. Electrochem., 2006, 36(10), 1163–1168 CrossRef CAS.
- X. H. Li, S. D. Deng, H. Fu and G. N. Mu, Inhibition action of tween-80 on the corrosion of cold rolled steel in sulfuric acid, Mater. Corros., 2009, 60(12), 969–976 CrossRef CAS.
- G. Perboni and G. Rocchini, Proceedings of the 10th ICMC, Madras, India, 1988, p. 2763 Search PubMed.
- O. Riggs, I. R. Hurd and M. Ray, Corrosion, 1967, 23(9), 252 CrossRef CAS.
- E. A. Noor and A. H. Al-Moubaraki, Corrosion Behavior of Mild Steel in Hydrochloric Acid Solutions, Int. J. Electrochem. Sci., 2008, 3, 806–818 CAS.
- T. P. Hoar and R. D. Holliday, The inhibition by quinolones and thioureas of the acid dissolution of mild steel, J. Appl. Chem., 1953, 3, 502 CrossRef CAS.
- G. M. Schmid and H. J. Huang, Spectro-electrochemical studies of the inhibition effect of 4,7-diphenyl-1,10-phenanthroline on the corrosion of 304 stainless steel, Corros. Sci., 1980, 20(8–9), 1041–1057 CrossRef CAS.
- M. A. Hegazy and M. F. Zaky, Inhibition effect of novel non-ionic surfactants on the corrosion of carbon steel in acidic medium, Corros. Sci., 2010, 52(4), 1333–1341 CrossRef CAS.
- K. J. Laidler, Reaction kinetics, Pergamon Press, New York, 1st edn, 1963, vol. 1 Search PubMed.
- N. Soltani, N. Tavakkoli, M. Khayatkashani, M. R. Jalali and A. Mosavizade, Green approach to corrosion inhibition of 304 stainless steel in hydrochloric acid solution by the extract of Salvia officinalis leaves, Corros. Sci., 2012, 62, 122–135 CrossRef CAS.
- M. Bouklah, B. Hammouti, M. Lagrenée and F. Bentiss, Thermodynamic properties of as a corrosion inhibitor for mild steel in normal sulfuric acid medium, Corros. Sci., 2006, 48, 2831–2842 CrossRef CAS.
- E. E. F. El-Sherbini, Effect of some ethoxylated fatty acids on the corrosion behaviour of mild steel in sulphuric acid solution, Mater. Chem. Phys., 1999, 60, 286–290 CrossRef.
- L. Xianghong and M. Guannan, Tween-40 as corrosion inhibitor for cold rolled steel in sulphuric acid: weight loss study, electrochemical characterization, and AFM, Appl. Surf. Sci., 2005, 252, 1254–1265 CrossRef.
- M. A. Quraishi and S. K. Shukla, Poly(aniline-formaldehyde): a new and effective corrosion inhibitor for mild steel in hydrochloric acid, Mater. Chem. Phys., 2009, 113, 685–689 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |