Exploiting the hydrophobicity of calixarene macrocycles for catalysis under “on-water” conditions†‡
Margherita De Rosa *,
Pellegrino La Manna,
Annunziata Soriente,
Carmine Gaeta,
Carmen Talotta and
Placido Neri*
*,
Pellegrino La Manna,
Annunziata Soriente,
Carmine Gaeta,
Carmen Talotta and
Placido Neri*
Dipartimento di Chimica e Biologia “A. Zambelli”, Università di Salerno, Via Giovanni Paolo II 132, I-84084 Fisciano, Salerno, Italy. E-mail: maderosa@unisa.it; neri@unisa.it
First published on 20th September 2016
Abstract
An example of calixarene-mediated catalysis under “on-water” conditions is here reported. In the presence of thioureido-calixarenes a rate acceleration for the Vinylogous Mukaiyama Aldol Reaction (VMAR) was observed, under on-water conditions. The ability of the calixarene catalyst to accelerate the VMAR under on-water conditions is closely related to its hydrophobicity and to its recognition abilities toward the substrate.
The title of a popular 1995 review by Böhmer suggested that calixarenes are “macrocycles with (almost) unlimited possibilities”.1 Thus, in the last twenty years a plethora of applications have been explored2 for them ranging from molecular recognition,3 sensing,4 and self-assembly,5 to the synthesis of interpenetrated architectures,6 and finally as catalysts.7
In the last years, many efforts have been devoted to the development of catalytic strategies environmentally oriented.8 In this regard, a particular emphasis has been placed on the study of organic reactions in water as medium.8 In a pioneering work Sharpless8b introduced the expression “on-water conditions” to denote the rate acceleration observed in organic reactions when insoluble reactants are vigorously stirred in H2O suspension. As highlighted by Sharpless,8b even if rate acceleration is negligible, the use of water presents interesting advantages such as the ease of product isolation and safety.
Under “on-water conditions” the supramolecular affinities between reactants and catalyst play a key role. In fact, it is known that the hydrophobic effect9 forces the reactants and the catalyst to aggregate and thus accelerating the reaction between them.10 In addition, the ability of an hydrophobic catalyst to act as hydrogen bond donor can be essential for the stabilization of the activated complex.10 Consequently, as recently reported by Song and Bae11 for squaramide-based catalysts, increasing the catalyst hydrophobicity can result in a significant rate acceleration.
Prompted by these considerations, we envisioned that the remarkable hydrophobicity of calixarene macrocycles12 could result in a rate acceleration if used as catalysts under “on-water conditions”. In addition, calixarene macrocycles present an hydrophobic cavity which can be readily adorned with supramolecularly interacting groups,12 such as amide, urea, or thiourea moieties, which could be useful for the substrate recognition. On this basis, we decided to investigate the abilities of calixarene macrocycles to act as catalysts under “on-water conditions”.13
Thus, we designed calixarene-based catalysts 1–5 (Fig. 1) to promote a Vinylogous Mukaiyama Aldol Reaction (VMAR)14 between furanone 6 and α-ketoesters 7 under “on-water conditions”. VMAR is one of the most useful methods in the art of making carbon–carbon bonds,14 particularly in the synthesis of complex organic compounds like polyketides15 and other natural products with various biological and pharmacological activities.16 Generally, VMAR is carried out in anhydrous organic solvents17 in the presence of promoters such as a Lewis acid or base.
Initially, we selected the combination of 2-(trimethylsiloxy)furan (TMSOF) 6 with methylbenzoylformate 7a (Scheme 1)18 to obtain butenolide architectures 8 and 9. We examined the feasibility of the reaction on-water without any catalyst at 30 °C (entry 1, Table 1). The reactants 6 and 7a were insoluble in the reaction medium, consequently, a rapid and vigorous magnetic stirring19 was applied. Under these conditions, the reaction proceeded sluggishly yielding only the γ-adduct 8a in poor yield after 24 h (Scheme 1).
| Entrya | Catalyst | Medium | Conv. to 8ab (%) | drc (anti/syn) |
|---|---|---|---|---|
| a All reactions were carried out using 0.2 mmol of methylbenzoylformate 7a, 1.2 equiv. of 6, 5.0 mol% of catalyst in 1 mL of medium at 30 °C.b Determined by 1H NMR analysis.c Determined by 1H NMR analysis according to literature data.20ad Deionized water.e Anhydrous solvent. The reactions were performed under rapid and vigorous magnetic stirring.19 | ||||
| 1 | — | H2Od | 25 | 63/37 |
| 2 | 1 | H2Od | 50 | 60/40 |
| 3 | 1 | CH2Cl2e | 31 | 40/60 |
| 4 | 1 | CH3OHe | 15 | 48/52 |
| 5 | 1 | THFe | 12 | 30/70 |
| 6 | 1 | Toluenee | Trace | nd |
| 7 | 2 | H2Od | 34 | 55/45 |
| 8 | 3 | H2Od | 23 | 50/50 |
When the reaction was performed in the presence of catalyst 1,18 under identical conditions, after 24 h the conversion of 7a raised up to 50% (entry 2, Table 1). An anti/syn ratio of 60/40 of the two diastereoisomers of γ-butenolide 8a was determined by integration of their 1H NMR signals,20a while no trace of the α-adduct 9a was observed. The stereochemical outcome of the VMAR in Scheme 1 can be rationalized through the models of the transition states proposed in Fig. 2. The hydrogen bonds between thiourea and carbonyl groups play a key role in the activation of the substrate 7 (Fig. 2a). Thus, the attack at the activated carbonyl group of 7a from the Re face of 6 results favored probably thanks to the stabilization induced by C–H⋯π (see inset in Fig. 2) interactions between methyl group of 2-(trimethylsilyloxy)furan 6 with aromatic walls of 1.
 | ||
| Fig. 2 (a) Plausible catalytic cycle for the VMAR catalyzed by calixarene derivatives 1 and 4. (b) and (c) Proposed transition states for the on water VMAR of 1 and 6 with α-ketoesters 7. | ||
At this point, following a known protocol,8b,11,19a we decided to compare the rate of the VMAR between 6 and 7a under identical conditions but in different solvents. When the reaction was carried out in organic solvents, where either 6, 7a, and calixarene-catalyst 1 are completely soluble, lower conversions (entries 3–6, Table 1) were observed. These data clearly indicates that calixarene-catalyst 1 is more active for VMAR under on-water conditions. Interestingly, in organic solvents we observed a switch of the stereoselectivity in favor of the syn adduct, with an anti/syn ratio of 40/60 and 30/70 in CH2Cl2 and THF (entries 3 and 5, Table 2), respectively.
| Entrya | 7a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratio (equiv.) 6 ratio (equiv.) |
T (°C) | t (h) | Conv. to 8ab (%) | drc (anti/syn) |
|---|---|---|---|---|---|
| a All reactions were carried out using 0.2 mmol of methylbenzoylformate 7a, 5.0 mol% catalyst in 1 mL of deionized water.b Determined by 1H NMR analysis.c Determined by 1H NMR analysis according to literature data.20a The reactions were performed under rapid and vigorous magnetic stirring.19 | |||||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
30 | 14 | 35 | 59/41 |
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
30 | 24 | 49 | 60/40 |
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
30 | 48 | 51 | 60/40 |
| 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
30 | 14 | 46 | 60/40 |
| 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
30 | 24 | 66 | 60/40 |
| 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
30 | 48 | 65 | 55/45 |
| 7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8 1.8 |
30 | 24 | 53 | 57/43 |
| 8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
50 | 24 | 66 | 59/41 |
| 9 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
50 | 14 | 66 | 60/40 |
In order to confirm the active role of the calix[4]arene framework for VMAR catalysis under on-water conditions, we performed the reaction between 6 and 7a in H2O in the presence of catalyst 2,18 which could be considered the closest analog of 1 devoid of the calix-macrocycle. Under analogous conditions (entry 7, Table 1) a 34% of conversion was obtained. These results clearly demonstrated that the hydrophobicity of the calixarene skeleton plays a fundamental role for the “on-water” catalysis of VMAR in Scheme 1.
Successively, we decided to investigate the role of the thiourea group in 1 in order to define its function for VMAR catalysis. When 6 and 7a were vigorously stirred in H2O at 30 °C for 24 h in the presence of calix[4]arene 3,21 depleted by the thiourea group at the exo-rim, a conversion of 23% (entry 8, Table 1) was obtained, significantly lower than that observed (50%) under analogous condition in the presence of 1 (entry 2, Table 1). These results clearly show that the thiourea group actively contributes to accelerate the VMAR rate, likely thanks to its ability to recognize 7a via H-bonding interactions.
To confirm this hypothesis, we investigated the binding affinity of 1 toward the substrate 7a by 1H NMR experiments in CDCl3.18,22 In details, a 1H NMR titration was performed18,23 in which the concentration of 1 was kept constant while the concentration of 7a was varied (Fig. S28‡).18 The addition of 7a to the solution of 1 caused significant downfield shifts of the NMR signals18 of NH thiourea protons (Fig. S28‡). This indicated that these groups were engaged in H-bonding interactions with the carbonyl group of 7a (Fig. 2) with a fast complexation equilibrium. A nonlinear least-squares fitting for a NH proton gives an association constant value of 600 ± 70 M−1 for the complexation of 7a with 1.18
Molecular mechanics calculations (AMBER force field) indicated that calixarene 1 (blue colored in Fig. 3) is able to complex α-ketoester 7a (in yellow, Fig. 3) by means of two H-bonds between the NH groups of 1 and the carbonyl group of 7a. In addition, the methyl group of 7a (in green, Fig. 3) was nested into the aromatic cavity of 1 establishing three stabilizing C–H⋯π interactions (mean distance C–H⋯πcentroid = 2.91 Å).
 | ||
| Fig. 3 Different views of the optimized structure of the 7a ⊂ 1 complex obtained by molecular mechanics calculations (AMBER force field). | ||
In order to optimize the reaction conditions, we studied the VMAR between 6 and 7a under on-water conditions by changing: temperature, reaction time, reagents molar ratio, calixarene-catalyst, and stirring method.
By prolonging the reaction time between 6 and 7a in the presence of 1, from 24 to 48 h, no further improvement of the yield was observed (entry 3, Table 2). Interestingly, when the 7a/6 molar ratio was changed from 1.0/1.2 to 1.0/1.5, then a significant increase (entry 5, Table 2) of the conversion of 7a was observed (66% vs. 49%). Moreover, an increase in the temperature of reaction shortened the reaction time from 24 to 14 h (entries 8 and 9, Table 2) leaving the selectivity unchanged.
With these results in hand, we turned our attention to the calixarene-size. As recently reported,11 the use of a more hydrophobic catalyst could result in a significant rate acceleration.
On this basis, we synthesized the larger thioureido-calix[6]arene catalyst 4,18 bearing hydrophobic moieties at both the exo and endo rim, such as tert-butyl and n-hexyl groups, respectively. When the reaction was performed in the presence of catalyst 4, an increase in the conversion up to 68% in 14 h was observed (entry 3, Table 3), whereas calix[4]-catalyst 1, required a 24 h reaction time to obtain a 66% of conversion (entry 2, Table 3), under the same conditions. This result clearly shows that by increasing the dimension of the calixarene-catalyst, and consequently its hydrophobicity, a significant increase of the VMAR rate occurs.
| Entrya | 7 | Catalyst | t (h) | Conv.b (%) | drc (anti/syn) |
|---|---|---|---|---|---|
| a All reactions were carried out using 0.221 mmol of 7a, 1.5 equiv. of 6, 5.0 mol% catalyst in 1 mL of deionized water at 30 °C.b Determined by 1H NMR analysis.c Determined by 1H NMR analysis according to literature data,20a while for the derivative 8c was ascertained by analogy.20b,c The reactions were performed under rapid and vigorous magnetic stirring.19 | |||||
| 1 | 7a | 1 | 14 | 46 | 57/43 |
| 2 | 7a | 1 | 24 | 66 | 54/46 |
| 3 | 7a | 4 | 14 | 68 | 80/20 |
| 4 | 7a | 4 | 24 | 72 | 70/30 |
| 5 | 7b | 1 | 14 | 36 | 67/33 |
| 6 | 7b | 4 | 14 | 18 | 57/43 |
| 7 | 7c | 1 | 14 | 31 | 54/46 |
| 8 | 7c | 4 | 14 | 36 | 50/50 |
| 9 | 7d | 4 | 14 | 42 | 50/50 |
In addition, an increase of the diastereoselectivity of VMAR between 6 and 7a in the presence of 4 was observed with an anti/syn ratio of 80/20 for 8a. Likely the presence of tert-butyl groups in 4 favors the attack of furanone 6 to the Re face of 7a (Fig. 4) thanks to the further stabilization induced by C–H⋯π interactions between tert-butyl groups of 4 and furan ring of 6 (inset in Fig. 4). Only slight changes in the conversion were obtained by prolonging the reaction time to 24 h (entry 4, Table 3). It is known that the stirring method affects the rate of organic reactions performed under on-water conditions.19 Therefore, we decided to perform the VMAR between 6 and 7a in the presence of 1 and 4 with a vortex stirring under otherwise identical conditions (at 30 °C, see Table 3). With this stirring method a 23 and 10% of conversions of 7a were observed after 14 h at 30 °C in the presence of catalysts 1 and 4 respectively, significantly lower with respect to the analogous magnetically stirred reactions (entries 1 and 3 in Table 3).
In analogy to 1, the reaction between 6 and 7a in the presence of 4 in organic solvents led to a dramatic decrease in the conversion percentages (Table 4) with respect to the on-water conditions. In these instances, an inversion of the VMAR diastereoselectivity was observed by switching from H2O (anti favored) to organic solvent (syn favored). Interestingly, these data indicated clearly that for both the catalysts 1 and 4 the syn/anti ratio observed for 8a is closely related to the nature of the solvent more than the structure of the calix-catalyst. Probably, in water the C–H⋯π interactions between 7a and the calixarene aromatic walls (see Fig. 2b and 4) are maximized thus favoring the anti 8a stereoisomer.
| Entrya | Medium | Conv.b (%) | drc (anti/syn) |
|---|---|---|---|
| a All reactions were carried out using 0.221 mmol of methylbenzoylformate 7a, 1.5 equiv. of 6, 5.0 mol% catalyst 4 in 1 mL of medium at 30 °C for 14 h.b Determined by 1H NMR analysis.c Determined by 1H NMR analysis according to literature data.20ad Deionized water.e Anhydrous solvent. | |||
| 1 | H2Od | 68 | 78/22 |
| 2 | CH2Cl2e | 39 | 32/68 |
| 3 | CH3OHe | 25 | 23/77 |
| 4 | THFe | Trace | nd |
| 5 | Toluenee | 10 | 34/66 |
Finally, when the VMAR between 6 and 7a was conducted in the presence of calix[6]-catalyst 5,24 which was depleted by the thiourea group at the exo-rim, only a 26% of conversion was obtained, with an anti/syn ratio of 60/40 for 8a. Thus, in analogy to what observed for 3, the H-bond recognition of 7a by thiourea group implies an acceleration rate of VMAR.
At this point, we decided to focus our attention to the α-ketoester substrate in order to evaluate the influence of its structure on the VMAR rate. When α-ketoester 7b, bearing an estereal ethyl group, was reacted with 6 in the presence of calix[4]arene-based catalyst 1, only a 36% of conversion was obtained after 14 h (entry 5, Table 3), while under analogous conditions the methyl α-ketoester analogue 7a was converted to 46% (entry 1, Table 3). Probably, the lower catalytic efficiency of 1 toward 7b is due to its lower affinity for the reactant.
To confirm this assumption, we performed a binding study between 1 and 7b by 1H NMR spectroscopy. As for 7a, an 1H NMR titration22 was performed in which the concentration of 1 was kept constant while the concentration of 7b was varied (Fig. S29‡). By these data, an association constant of 150 ± 30 M−1 for the complexation of 7b with 1 was calculated, a value significantly lower than that observed for the complexation of 7a with 1 (600 ± 70 M−1).
A close inspection of the optimized structure of 7a·1 complex (Fig. 2) suggests that probably the ethyl group of 7b is too large to be effectively hosted into the calix-cavity and consequently this involves less hydrophobic contacts between reactant 7b and catalyst 1, which justify the observed lower catalytic efficiency.
The above results clearly indicated that the ability of calix-catalyst 1 to accelerate VMAR under on-water condition is closely related to its recognition abilities toward the substrate 7, thus implying a supramolecular control of this catalysis.
In accordance with this conclusion, when the benzyl α-ketoester analogue 7c was reacted with 6 in the presence of 1 only a 31% of conversion was obtained. A similar trend was also observed with calix[6]arene-based catalyst 4, whereby increasing the size of the alcoholic moiety of the α-ketoester substrate 7 leads to a decrease of the catalyst efficiency.
In order to rationalize these data we have performed molecular mechanics calculations with the purpose to investigate the structure of the complexes between the calix[6]arene catalyst 4 and the α-ketoester substrates 7. Close inspection of the minimized (AMBER force field) structure (Fig. 5) of the complex between 4 and methyl ester 7a reveals that the methyl group of 7a is included in the aromatic cavity of 4 optimally oriented to establish a C–H⋯π interaction (distance C–H⋯πcentroid = 2.74 Å). In addition, H-bonds interactions were detected between the two NH groups of 4 and the carbonyl group of 7a (Fig. 5a), with a mean distance N⋯O of 2.78 Å and a mean angle N–H⋯O of 145.3°. Interestingly, the H-bonds in 7a·4 complex result shorter and stronger than those calculated for 7c·4 complex (Fig. 5c) in which a mean distance N⋯O of 2.87 Å and a mean angle N–H⋯O of 139.9° were found. Based on these results, we can conclude that 4 has the strongest ability to activate the carbonyl of 7a toward the nucleophilic attack of 6 respect to carbonyl group of 7c. Close inspection of the minimized structure of the 7c·4 complex reveals that the benzyl group is hosted into the cavity of 4 adopting an arrangement that facilitates the formation of C–H⋯π and π⋯π interactions (Fig. 5c), but less favorable to the formation of hydrogen bonds between N–H groups of 4 and carbonyl moiety of 7c. The data reported in Table 3 indicate a 42% of conversion of 7d after 14 h in presence of 4 a value higher than that observed for 7c under analogous conditions. Also in this case the higher catalytic efficiency towards the substrate 7d can be explained by the formation of stronger H-bonds between thiourea group of 4 and carbonyl group of 7d. In fact, examination of the minimized structure of the complex 7d·4 reveals the presence of two H-bonds N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C with a mean distance of 2.82 Å shorter than that observed for 7c (2.87 Å) and a mean angle N–H⋯O of 149.6°, more advantageous that the H-bond angle detected in 7c·4 complex (139.9°). In conclusions, analogously to catalyst 1 also for 4 the catalytic efficiency is closely related to the binding properties of the substrate 7 respect to the calixarene catalyst.
C with a mean distance of 2.82 Å shorter than that observed for 7c (2.87 Å) and a mean angle N–H⋯O of 149.6°, more advantageous that the H-bond angle detected in 7c·4 complex (139.9°). In conclusions, analogously to catalyst 1 also for 4 the catalytic efficiency is closely related to the binding properties of the substrate 7 respect to the calixarene catalyst.
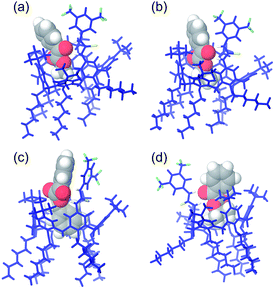 | ||
| Fig. 5 Optimized structures of the: (a) 7a·4, (b) 7b·4, (c) 7c·4 and (d) 7d·4 complexes obtained by molecular mechanics calculations (AMBER force field). | ||
Conclusions
In summary, we have explored the application of calixarene macrocycles as catalysts for a Vinylogous Mukaiyama Aldol Reaction (VMAR) under “on-water” conditions. We have found that the rate acceleration of the VMAR is closely related to the hydrophobicity of the calixarene skeleton and to its ability to recognize the α-ketoester via H-bonding interactions with the thiourea group. The results here reported can be considered an interesting example of supramolecular control of catalysis and could allow the development of new environmentally-oriented catalytic strategies. In these instances, the hydrophobicity and the synthetic versatility of calixarene macrocycles could be exploited for the design of novel benign catalysts.Notes and references
- V. Böhmer, Angew. Chem., Int. Ed. Engl., 1995, 34, 713 CrossRef.
- P. Neri, J. L. Sessler and M.-X. Wang, Calixarenes and Beyond, Springer, Switzerland, 2016 Search PubMed.
- (a) C. Gaeta, C. Talotta, F. Farina, M. Camalli, G. Campi and P. Neri, Chem.–Eur. J., 2012, 18, 1219 CrossRef CAS PubMed; (b) F. Troisi, A. Russo, C. Gaeta, G. Bifulco and P. Neri, Tetrahedron Lett., 2007, 48, 7986 CrossRef CAS; (c) F. Troisi, T. Pierro, C. Gaeta and P. Neri, Org. Lett., 2009, 11, 697 CrossRef CAS PubMed.
- N. Dozova, R. Kumar, T. Pradhan, F. Lacombat, B. Valeur, J. S. Kim and P. Plaza, Chem. Commun., 2015, 51, 14859 RSC.
- L. J. Prins, J. Huskens, F. de Jong, P. Timmermann and D. N. Reinhoudt, Nature, 1999, 398, 498 CrossRef CAS.
- C. Talotta, C. Gaeta and P. Neri, Org. Lett., 2012, 14, 3104 CrossRef CAS PubMed.
- (a) A. Soriente, M. Fruilo, L. Gregoli and P. Neri, Tetrahedron Lett., 2003, 44, 6195 CrossRef CAS; (b) A. Soriente, M. De Rosa, M. Fruilo, L. Lepore, C. Gaeta and P. Neri, Adv. Synth. Catal., 2005, 347, 816 CrossRef CAS; (c) S. Meninno, A. Parrella, G. Brancatelli, S. Geremia, C. Gaeta, C. Talotta, P. Neri and A. Lattanzi, Org. Lett., 2015, 17, 5100 CrossRef CAS PubMed; (d) Z.-Y. Li, J.-W. Chen, Y. Liu, W. Xia and L. Wang, Curr. Org. Chem., 2011, 15, 39 CrossRef CAS; (e) I. I. Stoikov, L. S. Yakimova, J. B. puplampu and A. A. Vavilova, in Organic Nanoreactors From Molecular to Supramolecular Organic Compounds, ed. S. Sadjadi, Academic Press Elsevier, London, 2016, ch. 4, pp. 85–110 Search PubMed.
- (a) R. Breslow, Acc. Chem. Res., 1991, 24, 159 CrossRef CAS; (b) S. Narayan, J. Muldoon, M. J. Finn, V. V. Fokin, H. Kolb and K. B. Sharpless, Angew. Chem., Int. Ed., 2005, 44, 3275 CrossRef CAS PubMed; (c) M. De Rosa and A. Soriente, Tetrahedron, 2011, 67, 5949 CrossRef CAS; (d) M. De Rosa, S. Di Marino, A. M. D'Ursi, M. Strianese and A. Soriente, Tetrahedron, 2012, 68, 3086 CrossRef CAS; (e) A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725 CrossRef CAS PubMed; (f) R. N. Butler and A. G. Coyne, Chem. Rev., 2010, 110, 6302 CrossRef CAS PubMed; (g) Y. Jung and R. A. Marcus, J. Am. Chem. Soc., 2007, 129, 5492 CrossRef CAS PubMed; (h) J. K. Beattie, C. S. P. McErlean and C. B. W. Phippen, Chem.–Eur. J., 2010, 16, 8972 CrossRef CAS PubMed.
- F. Biedermann, W. M. Nau and H.-J. Schneider, Angew. Chem., Int. Ed., 2014, 53, 11158 CrossRef CAS PubMed.
- J. E. Klijn and J. B. F. N. Engberts, Nature, 2005, 435, 746 CrossRef CAS PubMed.
- Y. H. Bae and C. E. Song, ACS Catal., 2015, 5, 3613 CrossRef.
- C. D. Gutsche, Calixarene, An introduction, RSC Publishing, 2nd edn, 2008 Search PubMed.
- (a) F. Biedermann, W. M. Nau and H. J. Schneider, Angew. Chem., Int. Ed., 2014, 53, 11158 CrossRef CAS PubMed; (b) E. Akceylan, A. Uyanik, S. Eymur, O. Sahin and M. Yilmaz, Appl. Catal., A, 2015, 499, 205 CrossRef CAS; (c) S. Sayin and M. Yilmaz, RSC Adv., 2014, 4, 2219 RSC; (d) P. Sarkar and C. Mukhopadhyay, Green Chem., 2016, 18, 442 RSC.
- (a) G. Casiraghi and F. Zanardi, Chem. Rev., 2000, 100, 1929 CrossRef CAS PubMed. For examples of catalytic strategies based on the use of calixarene catalysts in water, see: (b) S. Eymur, E. Akceylan, O. Sahin, A. Uyanik and M. Yilmaz, Tetrahedron, 2014, 70, 4471 CrossRef CAS; (c) S. Sayin and M. Yilmaz, RSC Adv., 2014, 4, 2219 RSC; (d) S. E. Denmark, J. R. Heemstra Jr and G. L. Beutner, Angew. Chem., Int. Ed., 2005, 44, 4682 CrossRef CAS PubMed; (e) G. Casiraghi, L. Battistini, C. Curti, G. Rassu and F. Zanardi, Chem. Rev., 2011, 111, 3076 CrossRef CAS PubMed; (f) S. V. Pansare and E. K. Paul, Chem.–Eur. J., 2011, 17, 8770 CrossRef CAS PubMed.
- (a) J. Hassfeld, M. Christmann and M. Kalesse, Org. Lett., 2001, 3, 3561 CrossRef CAS PubMed; (b) M. Kalesse, M. Cordes, G. Symkenberg and H.-H. Lu, Nat. Prod. Rep., 2014, 31, 563 RSC.
- L. Katz, Chem. Rev., 1997, 97, 2557 CrossRef CAS PubMed.
- The following examples have been reported in which a mixture of water/organic solvents was used: (a) L. Battistini, L. Dell'Amico, A. Sartori, C. Curti, G. Pelosi, G. Casiraghi, O. A. Attanasi, G. Favi and F. Zanardi, Adv. Synth. Catal., 2011, 353, 1966 CrossRef CAS; (b) H. Tian, Y. Chen, D. Wang, Y. Bu and C. Li, Tetrahedron Lett., 2001, 42, 1803 CrossRef CAS.
- See ESI for further details.‡.
- Previously reported data indicated that under on water conditions it is necessary a rapid and vigorous stirring of the reaction mixture in order to form an emulsion, see ref. 8b and: (a) J. K. Beattie, C. S. P. McErlean and C. B. W. Phippen, Chem.–Eur. J., 2010, 16, 8972 CrossRef CAS PubMed; (b) M. C. Pirrung, K. D. Sarma and J. Wang, J. Org. Chem., 2008, 73, 8723 CrossRef CAS PubMed.
- (a) M. Frings, I. Atodiresei, J. Runsink, G. Raabe and C. Bolm, Chem.–Eur. J., 2009, 15, 1566 CrossRef CAS PubMed; (b) C. Curti, L. Battistini, B. Ranieri, G. Rassu, G. Casiraghi and F. Zanardi, J. Org. Chem., 2011, 76, 2248 CrossRef CAS PubMed; (c) C. Curti, L. Battistini, F. Zanardi, G. Rassu, V. Zambrano, L. Pinna and G. Casiraghi, J. Org. Chem., 2010, 75, 8681 CrossRef CAS PubMed; (d) M. Woyciechowska, G. Forcher, S. Buda and J. Mlynarski, Chem. Commun., 2012, 48, 11029 RSC; (e) A. Adamkiewicz, M. Woyciechowska and J. Mlynarski, Eur. J. Org. Chem., 2016, 16, 2897 CrossRef.
- W. Verboom, S. Datta, Z. Asfari, S. Harkema and D. N. Rehinoudt, J. Org. Chem., 1992, 57, 5394 CrossRef CAS.
- When more polar H-bonds competitors solvent such as CD3CN and d6-DMSO were used no shift of the 1H NMR signals of 1 was observed (see Fig. S30‡).
- K. Hirose, in Analytical Methods in Supramolecular Chemistry, ed. C. A. Schalley, Wiley-VCH, Weinheim, 2007, pp. 17–54 Search PubMed.
- C. Gaeta, F. Troisi and P. Neri, Org. Lett., 2010, 12, 2092 CrossRef CAS PubMed.
Footnotes |
| † Dedicated to the memory of Carmela Spatafora. |
| ‡ Electronic supplementary information (ESI) available: Synthetic details, NMR and MS spectra, experimental procedures. See DOI: 10.1039/c6ra19270j |
| This journal is © The Royal Society of Chemistry 2016 |



