On peculiarities of Eu3+ and Eu2+ luminescence in Sr2GeO4 host
K. Fiaczyk and
E. Zych*
Faculty of Chemistry, University of Wroclaw, 14 F. Joliot-Curie Street, 50-383 Wroclaw, Poland. E-mail: Eugeniusz.zych@chem.uni.wroc.pl
First published on 13th September 2016
Abstract
Powders of Sr2GeO4:Eu were prepared in air or forming gas and were investigated for their luminescent properties in the 20–300 K range of temperatures. Oxidizing conditions produced a material with the orange-red luminescence of Eu3+. As many as eight Eu3+ symmetry sites active in luminescence were found at low temperatures. Four of them had the expected quite regular characteristics and were assigned to the four Sr sites offered by the host lattice. In addition, another four Eu3+ sites producing the 5D0 → 7F0 emission at extraordinarily short wavelengths in the range of 574–577 nm were clearly revealed. Their CT excitation bands peaked at around 325 nm, which is an unusually low energy for the O2− → Eu3+ CT transition. This unusual characteristic of Eu3+ luminescence was assigned to the presence of an interstitial oxygen not bound to Ge but strongly interacting with Eu3+ ions. The effect was justified using the Wybourne and Downer extension of the Judd–Ofelt theory. Reduced samples did not produce any Eu2+ emission at room temperature, but below about 70 K the 5d → 4f luminescence of the ion came into view with an intense appearance at 20 K. No indications were found of Eu2+ luminescence from a few sites. A possible explanation of this observation as well as mechanism of the Eu2+ luminescence quenching are discussed.
Introduction
Rare earth-activated Sr2SiO4 have been reported to be efficient luminescent materials.1–5 The violet-blue emission of Ce3+ and the green or yellow-orange (depending on the crystal structure) luminescence of Eu2+ were even considered useful for white LEDs.6–13 Thus, it is surprising that there is no literature on the luminescence of chemically quite similar Sr2GeO4:Eu. Despite the analogous chemical formulas, however, the germanate crystallizes in a different, orthorhombic crystal structure, space group Pbn21, offering as many as four different symmetry sites for Sr2+ ions.14 Both monoclinic and orthorhombic silicates, Sr2SiO4, offer just two Sr sites in their lattices.15,16The four different coordination spheres of Sr2+ sites in orthorhombic Sr2GeO4 are depicted in Fig. 1.14 Two of them are six-coordinated (Sr1 and Sr2) and the other two are eight-coordinated (Sr3 and Sr4). In Table 1 O2−–Sr2+ distances are listed.14 At first it is tempting to consider the two sites within each part (6- and 8-coordinated) similar in symmetry. However, closer analysis reveals that all four polyhedral sites are quite different and this should be mirrored by easily measurable differences in the position of their related f–f luminescence lines when substituted by rare earth luminescent ions. What the four sites have in common is their low local symmetries. Consequently, degeneracy of all the SLJ states with J ≠ 0 are expected to be lifted and much extended excitation and luminescence spectra with a longer reach are expected. Fig. 1 also presents a perspective view of the rather loosely packed unit cell.
 | ||
| Fig. 1 Coordination sphere of different sites for Sr2+ in orthorhombic Sr2GeO4. At the bottom a perspective view of the unit cell showing the rather loose packing of the structure is presented. | ||
| Sr1_CN6 | Sr2_CN6 | Sr3_CN8 | Sr4_CN8 | ||||
|---|---|---|---|---|---|---|---|
| O3 | 2.44 | O7 | 2.38 | O8 | 2.50 | O2 | 2.44 |
| O5 | 2.65 | O2 | 2.56 | O6 | 2.54 | O3 | 2.52 |
| O8 | 2.65 | O1 | 2.57 | O1 | 2.57 | O4 | 2.56 |
| O1 | 2.67 | O4 | 2.57 | O4 | 2.58 | O8 | 2.57 |
| O6 | 2.69 | O5 | 2.73 | O7 | 2.63 | O6 | 2.61 |
| O2 | 2.77 | O6 | 2.96 | O5 | 2.70 | O5 | 2.64 |
| — | — | — | — | O3 | 2.76 | O7 | 2.76 |
| — | — | — | — | O2 | 2.92 | O1 | 2.82 |
As we mentioned above, there is no literature data on the spectroscopic properties of a rare earth-activated Sr2GeO4 host. In the present paper we report on the photoluminescent properties of Sr2GeO4 doped with Eu3+ or Eu2+. In the Sr2GeO4 lattice the Eu2+ ion may readily substitute for Sr2+ because the ionic radii of Eu2+_CN6 (1.17 Å) and Eu2+_CN8 (1.25 Å) are almost identical with the sizes of Sr2+_CN6 (1.18 Å) and Sr2+_CN8 (1.26 Å), respectively.17 Yet, the ionic radii of Eu3+ ions are considerably (15–20%) smaller than Sr2+, reaching 0.95 Å for Eu3+_CN6 and 1.07 Å for Eu3+_CN8.17 On the other hand, the size of Eu3+ is more than double the size of Ge4+_CN4 (0.39 Å).17 Consequently, in practice, only the four Sr sites are supposed to be available for substitution by either Eu2+ or Eu3+ ions. Tracking this problem by means of photoluminescence spectroscopy is one of the purposes of this paper.
Experimental
Synthesis
Eu-doped Sr2GeO4 powders were prepared by the classic ceramic method. Concentrations of europium ions were 0.1, 0.5 and 2.0% mol. The ratios of the reagents were calculated assuming that europium substitutes for strontium in the host lattice. The substrates, SrCO3 (Alfa Aesar 99.995%), GeO2 (Alfa Aesar 99.9999%), Eu(NO3)3·6H2O (europium nitrate was prepared by dissolving Eu2O3 – Stanford Materials 99.999% purity in HNO3 – Chempur pure p.a.), were ground in an agate mortar and heated at 1500 °C for 3 h in air (for the Eu3+ emission) or in a reducing atmosphere of 25% H2 + 75% N2 forming gas also for 3 h to accomplish the Eu3+ → Eu2+ reduction.Measurements
Photoluminescence (PL), excitation spectra (PLE) and decay kinetics (DEC) were measured on a FLS980-sm Fluorescence Spectrometer from Edinburgh Instruments Ltd. A 450 W continuous xenon arc lamp as an excitation source for PL and PLE spectra and a 60 W xenon flash lamp and Fianium WhiteLase Supercontinuum Fiber Laser for DEC measurements were used. TMS302-X Single Grating excitation and emission monochromators of 30 cm focal length were used and the luminescence light was recorded by a Hamamatsu R928P high-gain photomultiplier detector. Emission spectra were corrected for the recording system spectral efficiency and excitation spectra were corrected for the incident light intensity. For all samples X-ray diffraction measurements were performed with a D8 Advance X-ray Diffractometer from Bruker in the range of 2θ = 20–50° with a step of 2θ = 0.01608°. Ni-filtered Cu Kα1 radiation (λ = 1.540596 Å) was utilized. A Hitachi S-3400N Scanning Electron Microscope (SEM) was used to image the microstructure of the powders.Results and discussion
Fig. 2 shows the measured XRD patterns of Sr2GeO4 powders with two different concentrations of europium activator – 0.1% and 2.0% – prepared in air or forming gas. The simulated pattern of the orthorhombic Sr2GeO4 is also given (ICSD#83345 (ref. 14)). Independent of the Eu concentration and preparation atmosphere, all powders are single phase orthorhombic materials. While the position of the measured and referenced diffraction lines match very well, some discrepancies in their intensity ratios are observed, especially around 2θ ∼ 31 degree. This may result from some preferential orientation of grains/crystallites, which may occur due to their significant aggregation and sintering even after pulverizing in a mortar (see Fig. 3). | ||
| Fig. 2 X-ray diffraction patterns of Sr2GeO4 containing 0.1% Eu or 2.0% of Eu and prepared in air and in reducing atmosphere of forming gas. | ||
 | ||
| Fig. 3 SEM images of powders of Sr2GeO4:0.1% Eu (a) and Sr2GeO4:2.0% Eu (b) fabricated in air and Sr2GeO4:0.1% Eu (c) and Sr2GeO4:2.0% Eu (d) prepared in a reducing atmosphere of 25% H2 + 75% N2. | ||
Fig. 3 presents SEM images of samples with different Eu concentrations and prepared in air or the forming gas. Fig. 3a and b depicts the morphology of Sr2GeO4 prepared in air atmosphere for low (0.1%) and high (2.0%) concentrations of activator, respectively. Alteration of the dopant content does not affect the morphology. Independent of the preparation atmosphere, aggregation of crystallites and their partial sintering is clearly observed. The size of the crystallites may be estimated at ∼2–3 μm for samples prepared in air. SEM images of samples containing 0.1% and 2% of Eu and prepared in the reducing atmosphere of the forming gas are presented in Fig. 3c and d, respectively.
The average sizes of their crystallites are clearly larger compared to powders prepared in air and reach roughly 4–10 μm. It is thus clear that the reducing atmosphere enhances the mass flow and thus facilitates the grain growth in Sr2GeO4, while the Eu content does not affect this process. Since treatment in the forming gas is expected to enhance the population of oxygen-vacancy sites,  , in the powder, it may be hypothesized that their appearance stimulates the host atoms' mobility and thus more significant grain growth is observed.18
, in the powder, it may be hypothesized that their appearance stimulates the host atoms' mobility and thus more significant grain growth is observed.18
Photoluminescence of Sr2GeO4:Eu3+
Each sample of air-heated Sr2GeO4:Eu shows the characteristic red Eu3+ emission upon excitation around 250–270 nm. More orange luminescence appears when the excitation moves towards 300–350 nm. Fig. 4 presents typical PLE spectra for Sr2GeO4:Eu3+ which show characteristic emissions and PL spectra recorded at RT and at 20 K. The RT PLE spectrum of the 588.6 nm luminescence (Fig. 4a, blue line) is composed of an O2− → Eu3+ charge transfer (CT) broad band with a maximum at about 260 nm and a set of narrow lines at longer wavelengths related to the f → f transitions of Eu3+.19,20 Thus, this spectrum is quite characteristic of Eu3+ in many other oxides. However, monitoring the 574.2 nm emission we get a quite different PLE spectrum with an intense CT band peaking at 325 nm and only weak lines related to the f → f transitions of Eu3+ (Fig. 4a, red line). The O2− → Eu3+ CT band located at such long wavelengths in oxides is quite peculiar. Yet, an analogous result was reported for α′-Sr2SiO4,21–23 Ba2SiO4,24 and La2Si2O7,25 which are not isostructural to Sr2GeO4. The corresponding PL spectra (Fig. 4b) cover the orange-red region from 570 nm to 720 nm and consist of a series of sharp lines due to the 5D0 → 7FJ (J = 0, 1, 2, 3, 4) transitions of Eu3+. Nevertheless, excitation into the two CT absorption bands (260 nm and 325 nm) produces quite different PL emissions. Excitation at 260 nm produces a classic Eu3+ luminescence with the main components related to the 5D0 → 7F1 (∼590 nm), 5D0 → 7F2 (∼620 nm) and 5D0 → 7F4 (∼700 nm). The 5D0 → 7F0 transition could not be unambiguously resolved, having a very low intensity. Also, transition to the 7F3 level produces only a very low-intensity emission around 650–660 nm. Under the 325 nm excitation the strongest luminescence line is observed at 574.2 nm, which is unusual for an Eu3+ ion. Different characteristics of the two Eu3+ emissions are further confirmed by PLE (Fig. 4c) and PL (Fig. 4d) spectra recorded at 20 K. Again, excitation around 325 nm produces Eu3+ luminescence with the dominant line positioned at 574.2 nm, while excitation at 257 nm gives quite a conventional spectral distribution for the Eu3+ emission. In both cases, the f–f related features in excitations and in emissions are much better resolved at 20 K than at RT, as expected.As a matter of fact, one might doubt whether the 574.2 nm emission results from the 5D0 → 7F0 transition. Yet, its decay time of τ = 1.0 ms (Fig. 5, red points) is identical to the decay times of the other emission features located at longer wavelengths and characteristic of the 325 nm excitation. This proves that all the observed emission lines upon 325 nm excitation result from the radiative relaxation of the same 5D0 level of the Eu3+ ion. The decay time of the characteristic luminescence features upon excitation around 260 nm reaches τ = 2.0 ms, as is seen in Fig. 5 (blue trace).
The above data disclosed two, spectroscopically very different, Eu3+ ions. Yet, the host offers, as we discussed, as many as four symmetry sites for the dopant. A closer look at the low-temperature emissions (Fig. 4d) makes it clear that the 5D0 → 7F1 transition upon 257 nm excitation produces more lines than might result for one Eu3+ site. Furthermore, the 5D0 → 7F0 emission feature around 574.2 nm (upon 325 nm excitation) is also split into at least 2 lines. Thus, despite the decay times of the various emissions giving only two different values (see Fig. 5), the low-temperature PL spectra actually indicate the presence of more emitting centers. Next, low-temperature and higher-resolution experiments will shed more light on that problem.
In the already-mentioned silicates, α-Sr2SiO4 (ref. 21) and Ba2SiO4,24 La2Si2O7,25 similarly peculiar Eu3+ luminescent lines around 574 nm, with all the characteristics of the 5D0 → 7F0 emission, were recorded. Their abnormally high energy was postulated to result from the presence of an extra interstitial O2− ligands not bound to silicon. However, other authors claimed that this 5D0 → 7F0 luminescence is merely related to Eu3+ located in a different crystallographic site.22,23 However, they did not offer a clear explanation as to why the 5D0 → 7F0 emission should appear at such short wavelengths.
5D0 → 7F0 luminescence around 574–576 nm was also reported for La0:95Eu0:05Ca4(PO4)3O, whose crystallographic structure may be derived from an apatite structure.26 In an analogous composition with Bi replacing La, the high-energy 5D0 → 7F0 luminescence was not present. It was concluded that the different electronic structure of Bi3+ vs. La3+ in the former reduced the covalent character of the Eu3+–O2− bond with oxygen not bound to P. Furthermore, in27 it was shown that in La3SbO7:Eu such a short-wavelength 5D0 → 7F0 luminescence is also observed while in Gd3SbO7:Eu it was not. Crystallographic considerations led to the conclusion that the more polarizable environment in the La-composition was responsible for that effect. Also in ref. 28 a narrow line around 575 nm is reported, although it is not discussed in depth. This may be attained when an interstitial oxygen atom is bound only to the emitting ion and/or the Eu3+ enters the site with a rather loose arrangement of ligands. In fact, both conditions are fulfilled in the case discussed here of Sr2GeO4.
To proceed with our analysis of the origin of the different Eu3+ emitting ions in Sr2GeO4, we performed additional more precise, higher-resolution experiments at 20 K to get a more comprehensive picture of that problem. The high-resolution experiments were performed for the whole range of wavelengths in which the various emissions appeared (570–720 nm) and exploiting 350–550 nm excitation range of the f–f transitions. But, in the following part of the paper only the most relevant fractions, where the differences are most clearly seen, are presented.
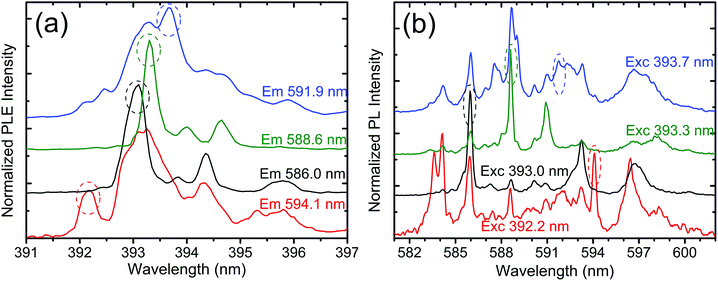
Fig. 6a shows PLE spectra of various lines related to the 5D0 → 7F1 transitions and corresponding PL spectra (Fig. 6b). Table 2 lists the characteristic excitation and emission lines and should be helpful in further considerations. Note that only lines unambiguously assigned to different sites are listed. In both PLE and PL spectra the features overlap. Despite the complexity it is clear from Fig. 6 that as many as four different excitation and emission spectra can be distinguished in the higher-resolution experiments at 20 K. Due to very low local symmetries of the emitting centers, we did not succeed in assigning the PL and PLE spectra to specific Eu symmetry sites. We do not exclude the possibility that it might be achievable with the help of computational chemistry.
Let us stress that they are all positioned within the typical range of wavelengths characteristic for Eu3+ transitions. While the achievable resolution is not sufficient to record fully site-selective spectra, with either PLEs or PLs, the differences observed within the range of the 5D0 → 7F1 transitions are adequate to conclude that as many as four Eu3+ ions of different local symmetries contribute to the luminescence in this range.
The above findings are remarkable. Already four Eu3+ emitting centers had been found within the regular set of PL lines, those best seen upon the nonselective excitation around 257 nm, see Fig. 4. Next lines shall be seen due to the high-resolution PLE and PL spectra focused on the peculiar emission around 574 nm efficiently excited around 325 nm (see Fig. 4).
Fig. 7 depicts the results of site-selective PLE and PL spectra of the 5D0 → 7F0 transitions with emission around 574 nm. PLE spectra are presented in the range of 458–464 nm (5D0 → 7F2 transitions, Fig. 7a) and PL emissions in the range of 572–579 nm (Fig. 7b). The results are straightforward. For the chosen excitation wavelengths, clearly distinct 5D0 → 7F0 emissions are observed. Their respective 7F0 → 5D2 excitation transitions are also significantly different and well-resolved. The positions of the characteristic lines are given in Table 2. Thus, for the peculiar luminescence with the CT excitation around 325 nm and 5D0 → 7F0 transitions at unusually short wavelengths, as many as four different Eu3+ emitting centers were also found.
Hence, as many as eight Eu3+ emitting centers were clearly resolved. They form two groups, each consisting of four Eu3+ sites sharing similar characteristics. The first group might be termed regular centers, as their emissions locate within the typical range of wavelengths for Eu3+ luminescence. The other four centers, let us term them irregular, are characterized by the peculiarly short wavelength of the 5D0 → 7F0 luminescence, located around 574–577 nm. Taking into account the characteristic properties of the centers, the regular ones should be assigned to the Eu3+ ions occupying the Sr2+ sites,  , and adopting their local symmetries in Sr2GeO4. This does not explain how the next four sites of Eu3+ are then formed. The answer to this question should also rationalize the peculiar spectroscopic characteristics of the four irregular sites.
, and adopting their local symmetries in Sr2GeO4. This does not explain how the next four sites of Eu3+ are then formed. The answer to this question should also rationalize the peculiar spectroscopic characteristics of the four irregular sites.
The low-energy (long-wavelength, peak around 325 nm) CT transition of the Eu3+ ions emitting around 574–577 nm may be understood by assuming that the dopant excessive charge is compensated by an interstitial O2− ligand,  , located in the first coordination sphere of the emitting ion. Since it would not be bound to Ge, its bond with Eu3+ would show a more covalent character than in the case of the regular oxygen atoms.29 This would, obviously, lower the energy needed to accomplish the
, located in the first coordination sphere of the emitting ion. Since it would not be bound to Ge, its bond with Eu3+ would show a more covalent character than in the case of the regular oxygen atoms.29 This would, obviously, lower the energy needed to accomplish the  → Eu3+ charge transfer transition, exactly as experimentally observed.
→ Eu3+ charge transfer transition, exactly as experimentally observed.
Furthermore, since  may in general locate randomly within the host lattice (though some tendency to trace positively charged
may in general locate randomly within the host lattice (though some tendency to trace positively charged  sites may occur by means of their coulombic interaction), only a fraction of all the Eu3+ ions encounter the extra
sites may occur by means of their coulombic interaction), only a fraction of all the Eu3+ ions encounter the extra  ligand in their first coordination sphere. And only Eu3+ ions within the
ligand in their first coordination sphere. And only Eu3+ ions within the 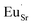 –
– associates would show the peculiar luminescent properties with the low-energy CT transitions and the short-wavelength 5D0 → 7F0 luminescence. Since both the regular and irregular sets of sites are composed of four Eu3+ centers it is apparent that Eu enters each of the four Sr sites in Sr2GeO4, and within each of them the
associates would show the peculiar luminescent properties with the low-energy CT transitions and the short-wavelength 5D0 → 7F0 luminescence. Since both the regular and irregular sets of sites are composed of four Eu3+ centers it is apparent that Eu enters each of the four Sr sites in Sr2GeO4, and within each of them the  interstices may be located.
interstices may be located.
The above explanation is consistent with the published data on similar specific luminescence of silicates – Sr2SiO4:Eu, Ba2SiO4:Eu and La2Si2O7:Eu.25 Obviously, the additional  ligand, not bound to Ge, around Eu3+ will strongly affect the position of the dopant electronic levels. This would be the privileged
ligand, not bound to Ge, around Eu3+ will strongly affect the position of the dopant electronic levels. This would be the privileged  –
–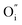 bond which would be responsible for the peculiar luminescence.
bond which would be responsible for the peculiar luminescence.
The peculiarly short wavelengths of the 5D0 → 7F0 transitions were often (though not always) connected with their abnormally high intensities. Sometimes they dominated the emission spectrum, although not always as strongly as in our case (see Fig. 4b and d). This is also curious as, according to the Judd–Ofelt theory, these transitions are strongly forbidden both as electric dipole and magnetic dipole transitions.30–33 Such an anomalously potent 5D0 → 7F0 luminescence of Eu3+, basically breaking the Judd–Ofelt theory rules, was reported for a number of compositions,34–36 among them Ba2SiO4,24 La2Si2O7,25 BiCa4(PO4)3,26 CuLaO2,37 BaFCl,38,39 Ba4Y2ZrWO12,40 Y3SbO7,27 LaOCl,41,42 LaOBr.43 The effect was also demonstrated in some apatites and their derivatives, like Ca10(PO4)6(OH)2, Ca10(PO4)6O,44 LaCa4(PO4)3O,26 M5(PO4)3X (M = Ca, Sr; X = F−, Cl−, Br).45 An important case is BaFCl:Eu3+ for which calculations were performed and it was shown that it is an oxygen-substituting F− in the first coordination sphere which is responsible for the irregular Eu3+ luminescence.38
It was noted that the irregular Eu3+ luminescence with the unusually high 5D0 → 7F0 emission intensity and energy often occurs in so-called charge-unbalanced materials, where the Eu3+ activator replaces a doubly charged metal ion site and when oxygen-compensating sites are likely to appear.39,46 Nevertheless, not all cases fall under this rule, which is clear from the above list of compositions. However, the strong 5D0 → 7F0 emission located at unusually high energy is regularly connected with a high energy of the 5D0 → 7F1 luminescence and large crystal field splitting of the 7F2 level.39 From Fig. 4d it is also clear that all these characteristics are presented by the irregular Eu3+ ions in Sr2GeO4. This observation clearly points to the importance of the effect that the local crystal field exerts on the irregular Eu3+ ions in their nearest neighborhood. Let us note that the O2− → Eu3+ CT represents a temporary reduction of the dopant to the Eu2+ state with a hole left behind at the oxygen atom. Then, the activator electron cloud expands altogether by about 20%,17 which necessarily leads to local distortion of the lattice and its substantial relaxation.
According to the Judd–Ofelt theory, the (so-called hypersensitive) 5D0 → 7F2 electric dipole (ED) transitions are sensitive to the local symmetry site, while the 5D0 → 7F1 magnetic dipole (MD) transitions are not. Consequently, their intensity ratios serve as a probe of the local symmetry site of the emitting Eu3+ ions. The Judd–Ofelt theory predicts that the 5D0 → 7F0 transition rate is always low. As was clarified in ref. 39, so-called J-mixing cannot account for such a spectacular increase in the 5D0 → 7F0 transition rate as is observed in the irregular Eu3+ luminescence. However, it has already been shown about 50 years ago that in the case of some particular site symmetries, allowing the operation of linear crystal field parameters, the 5D0 → 7F0 transition may rise greatly in intensity.47,48 This finally leads to an extension of the Judd–Ofelt theory proposed by Wybourne49–51 and Downer.52,53 As a result, it appears that even a small linear contribution to the crystal field Hamiltonian may boost the 5D0 → 7F0 transition probability if only the energy of charge transfer state is low enough.39 This occurs rarely, and is known as a third-order ED mechanism, which makes the 5D0 → 7F0 transition rate much higher than in regular cases and allows its intensity to even beat those of the other 5D0 → 7FJ components. This is the case we have in our Sr2GeO4:Eu and which is observed in other systems showing the irregular characteristics of the Eu3+ luminescence, which we cited above. On the other hand, the low-energy O2− → Eu3+ CT transition, peaking >∼300 nm, is not common but rather extraordinary. Its regular position is below ∼300 nm, often around 230–260 nm. In orthophosphates it occurs at even shorter wavelengths (higher energies). To lower the CT transition energy, at least one of the O2−-ligands has to be easily polarizable and its electrons should not be engaged into bonds with ions other than the emitting Eu3+. Then, the energy needed to move an electron from the O2−-ligand to the Eu3+ ion orbitals may be lower than in other cases. Consequently, the conditions for the third-order mechanism leading to the unusually significant intensity of the 5D0 → 7F0 luminescence as described by Wybourne and Downer are fulfilled.
There is thus every reason to claim that in Sr2GeO4:Eu the four lines around 574–577 nm which dominate the spectra of the irregular Eu3+ ions reflect the presence of a charge-compensating O2− ion in the first coordination sphere of Eu3+ dopants. This reduces the CT transition energy of that ion and in turn boosts the rate of the 5D0 → 7F0 transition well beyond those to the 7FJ levels with J > 0. What is quite unique in Sr2GeO4:Eu is that each of the four sites offered by the host experiences this effect. Thus the interstitial oxygen may locate in the first coordination sphere of each of the Eu3+ substituting either of the sites.
It is noteworthy that many of the host materials in which the 5D0 → 7F0 transitions of Eu3+ show these peculiar properties are rather loosely packed and/or contain chains formed by the M–O polyhedra, between which larger, unfilled spaces are present. These may be filled with oxygen interstices, facilitated when charge compensation is necessary. This is also true for Sr2GeO4:Eu3+, as indicated in Fig. 1. This further indicates that the occurrence of interstitial  sites in hosts in which their interaction is restricted to the emitting ions may well be the main source of the peculiar behaviour of the 5D0 → 7F0 transitions. In such specific, highly polarizable environments the higher rate of the 5D0 → 7F0 compared to other 5D0 → 7FJ transitions as well as its higher-energy locations were considered quite probable, as we have already mentioned above. Thus, the results presented in this paper and those already reported in the literature give the strong impression, quite well justified experimentally, that the abnormally high energy of the 5D0 → 7F0 transitions and/or their unusually high intensities are indeed connected with the presence of interstitial oxygen which is exclusively bonded/coordinated to the emitting center. Being easily polarizable, this oxygen lowers the energy of the CT transitions and also affects the rates of the f–f transitions.
sites in hosts in which their interaction is restricted to the emitting ions may well be the main source of the peculiar behaviour of the 5D0 → 7F0 transitions. In such specific, highly polarizable environments the higher rate of the 5D0 → 7F0 compared to other 5D0 → 7FJ transitions as well as its higher-energy locations were considered quite probable, as we have already mentioned above. Thus, the results presented in this paper and those already reported in the literature give the strong impression, quite well justified experimentally, that the abnormally high energy of the 5D0 → 7F0 transitions and/or their unusually high intensities are indeed connected with the presence of interstitial oxygen which is exclusively bonded/coordinated to the emitting center. Being easily polarizable, this oxygen lowers the energy of the CT transitions and also affects the rates of the f–f transitions.
Photoluminescence of Sr2GeO4:Eu2+
Samples prepared in a reducing atmosphere were expected to present a broad-band 5d → 4f Eu2+ luminescence. At room temperature, however, only a residual trace of Eu3+ luminescence could be recorded upon different excitations. This indicated that Eu was presumably reduced to Eu2+ (otherwise the Eu3+ luminescence would be strong), but the reduced form of the dopant does not emit light at RT. Indeed, after cooling the sample to 20 K, a broad-band intense emission peaking at 620 nm was recorded upon excitation at any wavelength in the range of 220–470 nm. See Fig. 8a. The emission band is assigned to the 4f65d1 → 4f7 (d → f) transition of Eu2+. The estimated Stokes shift reaches ∼7100 cm−1. This is quite a considerable value, which more than doubles the Stokes shift in BaMgAl10O17:Eu2+, a commercial blue phosphor.10,54 This is an important observation as a significant Stokes shift leads to significant thermal quenching.55Let us note that the long-wavelength excitation tail reaches zero-level intensity around 500 nm, while the short-wavelength tail of the emission onset appears around 530–535 nm, which gap already contributes ∼1200–1300 cm−1 to the Stokes shift. Such a characteristic of the Eu2+ luminescence points to a very significant relaxation occurring around the Eu2+ ion(s) after the absorption/excitation but before emission. This accords with the significant temperature quenching of the Eu2+ emission, well below 300 K as we shall see shortly. A higher Stokes shift of Eu2+ luminescence may also occur in so-called anomalous Eu2+ emission when the 4f → 5d excitation elevates the electron to the 5d level already immersed within the host conduction band (CB). There, taking advantage of the 5d-CB coupling the excited electron escapes its mother ion to localize on a nearby defect, giving an excitonic state with the hole left at the Eu. Relaxation of this entity produces the luminescent photons. This, however, typically leads to a broader emission with a longer decay time, and does not lead to a gap between excitation and emission spectra, as presented and discussed in Fig. 8. The decay time of our Sr2GeO4:Eu2+ at 45 K reaches a value of τ ∼ 1.5 μs and the luminescence band full width at half maximum (FWHM) attains 2550 cm−1 (100 nm). This value is barely larger than in the commercial BAM, in which the regular 5d → 4f Eu2+ luminescence has an FWHM of ∼2320 cm−1.54 Thus, we deduce that in Sr2GeO4:Eu2+ the emission is the regular 5d → 4f luminescence, and not the anomalous one.
As Fig. 9a shows, at 30 K the luminescence has already become slightly less intense and at 50 K its efficiency drops by more than 50% compared to the emission at 20 K. At 100 K the Eu2+ emission is almost totally gone. Furthermore, the decay trace at 100 K is strongly nonexponential (Fig. 9b) and its average value is as short as 〈τ〉 ∼ 80 ns (Table 3). With decreasing temperature, when the intensity of emission increases, the decay time gets longer and finally at 45 K its average value reaches 〈τ〉 ∼ 1.5 μs and the trace is almost monoexponential (Table 3).
| Temperature (K) | τ1 (ns) | Rel1% | τ2 (ns) | Rel2% | 〈τ〉 (ns) |
|---|---|---|---|---|---|
| 100 | 13.7 | 31.5 | 108.0 | 68.5 | 78.2 |
| 75 | 159.6 | 27.7 | 614.1 | 72.3 | 488.2 |
| 45 | 350.3 | 6.8 | 1631.3 | 93.2 | 1543.8 |
Such a low-temperature quenching is characteristic of a large shift between the parabolas representing the ground and the emitting states. This leads to their crossing, see Fig. 10a, close to the minimum of the emitting level parabola, which opens a nonradiative pathway for relaxation of the excited electrons already at low temperatures. However, the thermal quenching may also result from a proximity of the emitting level and the conduction band, see Fig. 10b. Then, an electron excited to the 5d level would be prone to escape to the conduction band if a sufficient amount of thermal energy were to become available. At present, we cannot offer an unequivocal explanation of which of the two mechanisms really occurs in Sr2GeO4:Eu2+ leading to the strong thermal quenching of the Eu2+ luminescence. Nevertheless, while the position of the Eu2+ luminescence in Sr2GeO4:Eu2+ (Fig. 8b) would be suitable to improve the colour rendering index in white LEDs,56 adding some red colour to the blue LEDs combined with the yellow YAG:Ce phosphor, and also the efficient f → d excitation around 440 nm would be very suitable, the thermal quenching documented and described above gives no hope for that.
 | ||
| Fig. 10 Simplified schemes of configuration coordinate diagrams explaining the postulated mechanisms of the Eu2+ luminescence thermal quenching due to a large Stokes shift of the ground and emitting state parabolas. At low temperatures radiative relaxation occurs. At higher temperatures the thermal population of higher vibronic states of the emitting level (blue line) allows for non-radiative flow of the electrons down to the ground electronic state (red line) over the parabolas' crossing point (a).57 (b) shows an alternative thermal quenching mechanism with the emitting level located only slightly below the host conduction band (CB) to which the electron may escape at higher temperatures, precluding its radiative relaxation. See text. | ||
Independent of the Eu content, for Eu2+ luminescence we do not see any indications of emission from different sites. This is in contrast to the materials doped with Eu3+ for which all four sites could be identified in Eu3+ emissions and they were even doubled due to the interstitial oxygen in the first coordination sphere of Eu3+, as discussed earlier. It is also different from Sr2SiO4:Eu, in which two well-separated Eu2+ emissions were reported.58 However, since the silicate is not isostructural with the germinate,14 any deeper comparisons are not in fact substantiated. A speculative explanation of such behavior of Sr2GeO4:Eu2+ might be that excited 5d levels of all but one type of Eu2+ ion in the host are located within its conduction band. Consequently, only the dopants whose first excited 5d level locates below the conduction band would contribute to the d → f emission at sufficiently low temperatures. Those in other sites would be blind at any temperatures.
Conclusion
Orthorhombic powders of Sr2GeO4 doped with either Eu3+ or Eu2+ ions were prepared by means of the ceramic method in air or forming gas, respectively. XRD studies confirmed the formation of an orthorhombic phase, space group Pbn21, independent of the oxidation state of Eu and their concentration. The luminescence studies of the Sr2GeO4:Eu3+ revealed that all four available sites offered by the host are occupied by Eu3+ and contribute to the luminescence. All of those sites produce a typical red luminescence of Eu3+ with CT excitation peaking around 260 nm. However, some of the dopant ions give emission with the 5D0 → 7F0 luminescence located around 574 nm, which is an unusually short wavelength for that transition. Another peculiarity is that these Eu3+ ions show the CT excitation transition peaking around 325 nm, which is an exceptionally long wavelength for oxide hosts. Such behavior was explained by the presence of an interstitial oxygen, , bound to Eu3+ by a strongly covalent interaction, which was possible because, contrary to the regular O-atoms, the
, bound to Eu3+ by a strongly covalent interaction, which was possible because, contrary to the regular O-atoms, the  was not bound to the Ge. The effect was discussed with the help of the Wybourne and Downer extension to the Judd–Ofelt theory. Eu2+ ions show their allowed 5d → 4f emission only below 100 K. The reason for such a strong thermal quenching was postulated to come from either a nonradiative relaxation because of a strong shift between ground and emitting states parabolas or from low-energy separation of the emitting 5d level and the host conduction band. Unfortunately, the spectroscopic characteristics of both the Sr2GeO4:Eu3+ and Sr2GeO4:Eu2+ preclude their applicability in phosphor-converted white LEDs.
was not bound to the Ge. The effect was discussed with the help of the Wybourne and Downer extension to the Judd–Ofelt theory. Eu2+ ions show their allowed 5d → 4f emission only below 100 K. The reason for such a strong thermal quenching was postulated to come from either a nonradiative relaxation because of a strong shift between ground and emitting states parabolas or from low-energy separation of the emitting 5d level and the host conduction band. Unfortunately, the spectroscopic characteristics of both the Sr2GeO4:Eu3+ and Sr2GeO4:Eu2+ preclude their applicability in phosphor-converted white LEDs.
Acknowledgements
The authors are indebted to Dr Joanna Cybinska for helpful discussions. This work was supported by POIG.01.01.02-02-006/09 project co-funded by European Regional Development Fund within the Innovative Economy Program. Priority I, Activity 1.1. Subactivity 1.1.2.References
- S. H. M. Poort, W. Janssen and G. Blasse, J. Alloys Compd., 1997, 260, 93–97 CrossRef CAS.
- J. S. Lee and Y. J. Kim, J. Nanosci. Nanotechnol., 2012, 12, 8630–8634 CrossRef CAS PubMed.
- J. K. Park, M. A. Lim, C. H. Kim, H. D. Park, J. T. Park, S. Y. Choi, J. K. Park, A. Lim, C. H. Kim and H. D. Park, Appl. Phys. Lett., 2011, 82, 683–685 CrossRef.
- L. Zhang, Z. Lu, P. Han, J. Lu, N. Xu, L. Wang and Q. Zhang, J. Am. Ceram. Soc., 2012, 95, 3871–3877 CrossRef CAS.
- J. Y. Woo, K. Kim, S. Jeong and C. S. Han, J. Phys. Chem. C, 2011, 115, 20945–20952 CAS.
- Z.-J. Wang, Z.-P. Yang, Q.-L. Guo, P.-L. Li and G.-S. Fu, Chin. Phys. B, 2009, 18, 2068–2071 CrossRef CAS.
- X. Sun, J. Zhang, X. Zhang, Y. Luo and X. Wang, J. Rare Earths, 2008, 26, 421–424 CrossRef.
- M. A. Tshabalala, F. B. Dejene, S. S. Pitale, H. C. Swart and O. M. Ntwaeaborwa, Phys. B, 2014, 439, 126–129 CrossRef CAS.
- J. K. Park, K. J. Choi, C. H. Kim, H. D. Park and S. Y. Choi, Electrochem. Solid-State Lett., 2004, 7, H15 CrossRef CAS.
- C. Ronda, Luminescence From Theory to Applications, WILEY-VCH, Weinheim, 2008 Search PubMed.
- T. Q. Khanh, P. Bodrogi, Q. T. Vinh and H. Winkler, LED Lighting, Technology and Perception, Weinheim, 2015 Search PubMed.
- R. Xie, N. Hirosaki, T. Suehiro and F. Xu, Chem. Mater., 2006, 18, 5578–5583 CrossRef CAS.
- Y. Q. Li, A. C. A. Delsing, G. De With and H. T. Hintzen, Chem. Mater., 2005, 17, 3242–3248 CrossRef CAS.
- F. Nishi and Y. Takeuchi, Z. Kristallogr., 1996, 211, 607–611 CAS.
- M. Catti, G. Gazzoni, G. Ivaldi and G. Zanini, Acta Crystallogr., Sect. B: Struct. Sci., 1983, 39, 674–679 CrossRef.
- M. Catti and G. Gazzoni, Acta Crystallogr., Sect. B: Struct. Sci., 1983, 39, 679–684 CrossRef.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- W. D. Kingery, H. K. Bowen and D. R. Uhlmann, Introduction to Ceramics, John Wiley & Sons, 2nd edn, 1976 Search PubMed.
- G. H. Dieke and H. Crosswhite, Spectra and energy levels of rare earth ions in crystals, Interscience Publishers Wiley, New York, USA, 1968 Search PubMed.
- J. Zeler, J. Cybińska and E. Zych, RSC Adv., 2016, 6, 57920–57928 RSC.
- E. D. Bacce, A. M. Pires and M. R. Davolos, J. Alloys Compd., 2002, 344, 312–315 CrossRef CAS.
- S. K. Gupta, M. Mohapatra, S. Kaity, V. Natarajan and S. V Godbole, J. Lumin., 2012, 132, 1329–1338 CrossRef CAS.
- A. Nag and T. R. N. Kutty, J. Mater. Chem., 2004, 14, 1598–1604 RSC.
- A. M. Pires, M. R. Davolos and O. L. Malta, J. Lumin., 1997, 74, 244–246 CrossRef.
- B. Piriou, M. Richard-Plouet, J. Parmentier, F. Ferey and S. Vilminot, J. Alloys Compd., 1997, 262–263, 450–453 CrossRef CAS.
- N. Lakshminarasimhan and U. V. Varadaraju, J. Solid State Chem., 2004, 177, 3536–3544 CrossRef CAS.
- J. Wang, Y. Cheng, Y. Huang, P. Cai, S. Il Kim and H. J. Seo, J. Mater. Chem. C, 2014, 2, 5559–5569 RSC.
- H. Xie, J. Lu, Y. Guan, Y. Huang, D. Wei and H. J. Seo, Inorg. Chem., 2014, 53, 827–834 CrossRef CAS PubMed.
- A. M. Srivastava, Opt. Mater., 2016, 58, 89–92 CrossRef CAS.
- G. S. Ofelt, J. Chem. Phys., 1962, 37, 511–520 CrossRef CAS.
- B. R. Judd, Phys. Rev., 1962, 127, 750–761 CrossRef CAS.
- B. G. Wybourne, J. Alloys Compd., 2004, 380, 96–100 CrossRef CAS.
- P. A. Tanner, Y. Y. Yeung and L. Ning, J. Phys. Chem. A, 2013, 117, 2771–2781 CrossRef CAS PubMed.
- L. Smentek and A. Kȩdziorski, J. Alloys Compd., 2009, 488, 586–590 CrossRef CAS.
- A. S. Souza, Y. A. R. Oliveira and M. A. Couto Dos Santos, Opt. Mater., 2013, 35, 1633–1635 CrossRef CAS.
- A. K. Parchur and R. S. Ningthoujam, RSC Adv., 2012, 2, 10859 RSC.
- F. Fujishiro, M. Murakami, T. Hashimoto and M. Takahashi, J. Ceram. Soc. Jpn., 2010, 118, 1217–1220 CrossRef CAS.
- X. Y. Chen, W. Zhao, R. E. Cook and G. K. Liu, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 1–9 CrossRef.
- X. Y. Chen and G. K. Liu, J. Solid State Chem., 2005, 178, 419–428 CrossRef CAS.
- H. Xie, L. Xu, L. Qin, Y. Huang, D. Wei, S. Il Kim and H. J. Seo, Mater. Lett., 2014, 115, 18–20 CrossRef CAS.
- G. Blasse and A. Bril, J. Chem. Phys., 1967, 46, 2579 CrossRef CAS.
- J. Holsa and P. Porcher, J. Chem. Phys., 1981, 75, 2108 CrossRef CAS.
- J. Holsa and P. Porcher, J. Chem. Phys., 1982, 76, 2790 CrossRef CAS.
- B. Piriou, D. Fahmi, J. Dexpert-Ghys, A. Taitai and J. L. Lacout, J. Lumin., 1987, 39, 97–103 CrossRef CAS.
- R. Jagannathan and M. Kottaisamy, J. Phys.: Condens. Matter, 1995, 7, 8453–8466 CrossRef CAS.
- L. Gaigerova, O. F. Dudnik, V. F. Zolin and V. A. Kudryashova, Luminescence of Crystals, Molecules and Solutions, Plenum Press, New York, 1973 Search PubMed.
- W. C. Nieuwpoort and G. Blasse, Solid State Commun., 1966, 4, 227 CrossRef CAS.
- B. R. Judd, J. Chem. Phys., 1966, 44, 839 CrossRef CAS.
- B. G. Wybourne, Optical Properties of Ions in Crystals, Wiley Interscience, New York, 1967 Search PubMed.
- B. G. Wybourne, J. Chem. Phys., 1968, 48, 2596 CrossRef.
- B. G. Wybourne, Eur. J. Solid State Inorg. Chem., 1991, 28, 19 CAS.
- M. C. Downer, G. W. Burdick and D. K. Sardar, J. Chem. Phys., 1988, 89, 1787–1797 CrossRef CAS.
- G. W. Burdick, M. C. Downer and D. K. Sardar, J. Chem. Phys., 1989, 91, 1511 CrossRef CAS.
- E. Zych, W. Goetz, N. Harrit and H. Spanggaard, J. Alloys Compd., 2004, 380, 113–117 CrossRef CAS.
- G. Blasse and B. C. Grabmaier, Luminescent Materials, Springer-Verlag, 1994, vol. 1 Search PubMed.
- P. Mottier, LED for Lighting Applications, Wiley-ISTE, 2010 Search PubMed.
- E. Zych, Inorganic display materials, in Handbook of luminescence, display materials, and devices, 2003, vol. 2, pp. 251–300 Search PubMed.
- A. Madej and E. Zych, RSC Adv., 2015, 5, 104441–104450 RSC.
| This journal is © The Royal Society of Chemistry 2016 |