A luminescent metal–organic framework for highly selective sensing of nitrobenzene and aniline†
Xin-Lei Huang,
Lin Liu,
Ming-Liang Gao and
Zheng-Bo Han*
College of Chemistry, Liaoning University, Shenyang 110036, P. R. China. E-mail: ceshzb@lnu.edu.cn
First published on 6th September 2016
Abstract
A stable nonanuclear rare earth carboxylate cluster based metal–organic framework, (DMA)2[Y9(μ3-OH)8(μ2-OH)3BTB6]n·(solv)x (gea-MOF-1) (DMA = dimethylamine cation and BTB = 1,3,5-benzene(tris)benzoate) can act as a fluorescent sensor for highly sensitive and selective detection of nitrobenzene and aniline through fluorescence quenching. More importantly, the detection limit of nitrobenzene is on the 5–60 ppm scale.
Efficient and fast detection of explosives, poisonous ions and small organic molecules is of current interest for a variety of reasons, including national security and environmental concerns.1 Nitrobenzene (NB) is generally used as the major vital component of commercial explosives which is a typical organic pollutant.2 Aniline (AN) plays an important role in organic synthesis and is used as a dye intermediate, pesticide and stabilizer of explosives. On account of its toxicity, it could mainly cause methemoglobinemia, hemolytic anemia, etc.3 Therefore, the identification and detection of these explosives and hazardous substances are important for environmental protection and human health. In recent years, there have been many analytical methods to monitor these compounds, for example, gas chromatography-mass spectrometry, Raman spectroscopy, ion mobility spectrometry and fluorescence, etc.4 Compared with other analytical techniques, fluorescence is more attractive because of its simplicity and high sensitivity, has broad applications for these organic molecular recognition and sensing.5
Metal–organic frameworks (MOFs) have attracted widespread interest in recent years, because of their delicate architectures, topological diversity, controllable channels and attractive potential applications such as gas adsorption, catalysis and sensing.6 More and more rare earth (RE) MOFs have been synthesized which were usually applied as fluorescence sensor.7 Zhang et al. reported a robust microporous luminescent Tb-MOF, [Tb(BCB)(DMF)](DMF)1.5(H2O)2 for the highly selective sensing of aniline.8 Our group synthesized lanthanide–organic frameworks [Tb(mtpc)1.5(DMA)(H2O)]·2H2O, could be applied as a fluorescence sensor for the detection of nitrobenzene.9
According to the previously reported lietrature,10 (DMA)2[Y9(μ3-OH)8(μ2-OH)3BTB6]n·(solv)x (DMA = dimethyl amine cation and BTB = 1,3,5-benzene(tris)benzoate) gea-MOF-1 was synthesized and confirmed by powder X-ray diffraction (PXRD) patterns (Fig. S1, ESI†). This porous MOF material exhibits excellent chemical stability and has the potential for heterogeneous catalysis application. In our previous work, gea-MOF-1 shows prominent fluorescent property for sensing Fe(III) ions.11 In this work, the gea-MOF-1 material also displays excellent fluorescence performance for sensing of NB and AN, which demonstrates the great potential of gea-MOF-1 as a highly selective multi-responsive fluorescence sensor.
Based on the previous research, gea-MOF-1 is a (3,18)-connected MOF with an unusual gea topology and nonanuclear carboxylate cluster serve as an 18-connected molecular building block for the assembly of a (3,18)-connected MOF with an unusual gea topology. It exhibits three distinct cages (van der Waals (vdW) distances: cavity I, 22.4 Å × 22.4 Å; cavity II, 24.8 Å × 14.6 Å; cavity III, 11.2 Å × 5.6 Å), cavity I being seen as a channel (aperture, vdW distances 12.8 Å × 9.4 Å) (Fig. 1).
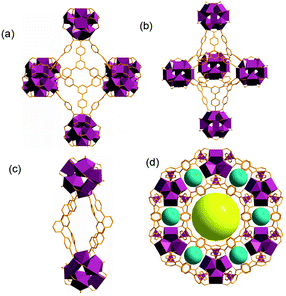 | ||
| Fig. 1 Open spaces and general view of gea-MOF-1 reveal three types of cavity, (a) cavity I, (b) cavity II, (c) cavity III, (d) cavity I being seen as a channel. | ||
Gea-MOF-1 contains aromatic or conjugated π moieties that are promising candidates for potential photoactive materials, which inspire us to systematically explore its potential application in this field.12 We studied the solid state photo-luminescent properties of H3BTB and gea-MOF-1 (Fig. S2 and S3, ESI†). Gea-MOF-1 displayed a strong solid-state luminescence at 373 nm upon excitation at 290 nm at room temperature, exhibiting a blue shift with respect to the free H3BTB (385 nm), which can be tentatively attributed to π → π* transition of the intraligand. In order to investigate luminescence performance, the powder sample of gea-MOF-1 was immersed in methanol, ethanol, acetone, acetonitrile (MeCN), tetrahydrofuran (THF), respectively. Treated by ultrasonication and then aged to generate stable suspensions before the fluorescence study. Among these small solvent molecules, the fluorescence intensity of gea-MOF-1 in methanol is obvious stronger than other solvents (Fig. S4, ESI†). So the followed fluorescent detection experiments were carried out in the methanol suspension of gea-MOF-1. As previously reported, gea-MOF-1 showed chemical stability in MeCN, THF, acetone, methanol, ethanol solvents.10
Meanwhile, the capacity of gea-MOF-1 for selective sensing of aromatic compounds was studied. Some aromatic compounds with the same concentration (60 ppm), such as benzene (BZ), toluene (TO), phenol (PhOH), nitrobenzene (NB), chlorobenzene (Cl-BZ), bromobenzene (Br-BZ), iodobenzene (I-BZ), nitrobenzene (NB), o-xylene (OX), m-xylene (MX), p-xylene (PX) and ethyl-benzene (E-BZ) were added to the methanol suspension of gea-MOF-1, respectively (Fig. 2). The result indicates that only NB causes an obvious quenching effect on the fluorescence intensity of methanol suspension of gea-MOF-1. Thus, gea-MOF-1 could be applied as a fluorescence sensor for the detection of NB.
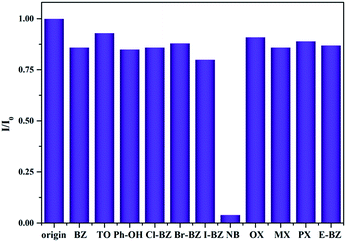 | ||
| Fig. 2 Room-temperature fluorescent intensity of gea-MOF-1 at 373 nm in methanol suspension of gea-MOF-1 with 60 ppm of different aromatic compounds (λex = 290 nm). | ||
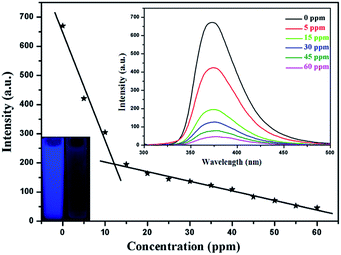
Further studies on exploring the detection limit of NB were carried out. As shown in Fig. 3, the fluorescence quenching degree decreased gradually with the reducing of the NB concentration. When the concentration of NB was 5 ppm, the quenching efficiency reached 37.2%. This phenomenon gives evidence to that gea-MOF-1 possesses excellently high sensitivity toward NB. When the concentration of NB was 60 ppm, the fluorescence was almost quenched completely. For comparison, the quenching efficiency of some fluorescent MOFs sensors for NB is summarized (Table S1, ESI†).13 The quenching efficiency of gea-MOF-1 is higher than other MOFs sensors for NB in Table S1, ESI.† Additionally, we investigated the potential of gea-MOF-1 towards sensing a series of nitroaromatic compounds in more detail, such as 2,4-dinitrophenol (DNP), p-nitrophenol (PNP), 4-nitrotoluene (4-NP), o-nitrophenol (ONP). The order of quenching efficiencies for the selected nitroaromatic compounds at the same concentration with 60 ppm was NB (93.1%) > ONP (18.1%) > DNP (15.8%) > 4-NP (14.0%) > PNP (11.3%) (Fig. S5, ESI†), indicating that gea-MOF-1 displays the most effective detection for NB among the different nitroaromatic compounds. The selectivity of NB in the presence of other nitroaromatic compounds was also studied.14 A specially designed experiment were performed by addition of different nitroaromatic compounds followed by NB into the methanol suspension of gea-MOF-1, and the corresponding emission spectra were monitored. The initial addition of different nitroaromatic compounds showed a negligible effect on the fluorescence intensity of gea-MOF-1. While, with the addition of NB to nitroaromatic compounds-containing solution, gea-MOF-1 gave significant fluorescence quenching, with the quenching efficiency of NB remaining unaffected. The stepwise decrease in the fluorescence intensity demonstrates the high ability of gea-MOF-1 for selectively detecting NB, even in the presence of other nitroaromatic compounds (Fig. S6, ESI†). Meanwhile, we also investigated the effect of some aromatic compounds on the anti-interference ability of gea-MOF-1 (Fig. S7, ESI†). The results suggest that gea-MOF-1 has outstanding anti-interference ability.
Furthermore, we also found that gea-MOF-1 can be regenerated and reused for a significant number of cycles by centrifugation of the solution after use and washing several times with fresh methanol. The quenching efficiencies of every cycle are basically unchanged at about 90%, through monitoring the emission spectra of gea-MOF-1 dispersed in the presence of 60 ppm NB in methanol from cycle 1 to 5 (Fig. S8, ESI†). These results clearly indicate that gea-MOF-1 shows high recyclability. The PXRD pattern further confirms that the framework is retained after cycle 5 (Fig. S11, ESI†).
The mechanism of quenching effect has been discussed. Gea-MOF-1 is highly sensitive to NB, but show low sensitivity to other nitroaromatic compounds and aromatic compounds. To deeply understand the fluorescent quenching effect by NB, the UV-Vis spectra were measured in Fig. S12, ESI,† the absorption band of NB partially overlaps with the absorption band of gea-MOF-1. However, no obvious absorption bands are found for other nitroaromatic compounds. Obviously, the overlap of the absorption bands causes a competition for excitation energy between NB and gea-MOF-1, such competitive adsorption will significantly decrease the transfer of excitation energy. This result shows the energy transfer mechanism is predominant in fluorescence quenching by NB.15
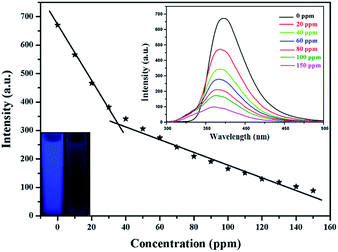
We also investigated the potential performance of gea-MOF-1 for the sensing of amines. Some organic amines with the same concentration (150 ppm), such as ammoniawater (AW), N,N-dimethylethylamine (DMAC), ethylenediamine (EDA), triethylamine (TEA), n-butylamine (BA), aniline (AN), N,N-dimethylformamide (DMF), N,N-dimethylacetamide (DMA) were added to the methanol suspension of gea-MOF-1, respectively. As shown in Fig. 4, gea-MOF-1 has a selective fluorescence response to AN through obvious fluorescence quenching, providing a possibility of gea-MOF-1 being a wonderful fluorescence sensor for AN. To examine the sensing sensitivity towards AN in more detail, the different amounts of AN were added to the methanol suspension of gea-MOF-1. As shown in Fig. 5, when the concentration of AN was 20 ppm, the quenching efficiency reached 30.4%. When the concentration of AN was about 150 ppm, the fluorescence was almost quenched completely. These results show that gea-MOF-1 could be applied as a fluorescence sensor for AN. The anti-interference sensing ability of gea-MOF-1 was performed by the competing experiments (Fig. S9, ESI†). These results also displayed that gea-MOF-1 shows outstanding anti-interference ability and could be used for sensing in the systems with complicated components. In addition, the recycling experiments were further carried out. Gea-MOF-1 was recovered by washing several times with fresh methanol after quenching and then reused. The quenching efficiencies are basically unchanged during 5 cycles of recyclability tests, indicating gea-MOF-1 exhibited high recyclability for sensing of AN (Fig. S10, ESI†). The PXRD pattern further confirms that the framework is retained after cycle 5 (Fig. S11, ESI†). In the present work, gea-MOF-1 is highly sensitive to AN, but show almost no sensitivity to aliphatic amines. The possible mechanism of quenching effect has been discussed. The strong supramolecular interactions, such as aromatic stacking effects between AN and BTB in the framework, as well as hydrogen bonding between amino groups of AN and water molecules coordinated to Y(III) ions, might further enhance the electron transfer.8,16 Furthermore, the UV-vis absorption (see Fig. S12, ESI†), the absorption bands of AN is almost overlapped by the wide absorption bands of gea-MOF-1, which would reduce the absorption of light by gea-MOF-1 and affected the energy conversion process in the host framework. Based on the above evidences, fluorescence intensities finally lead to a decrease or even quenching.
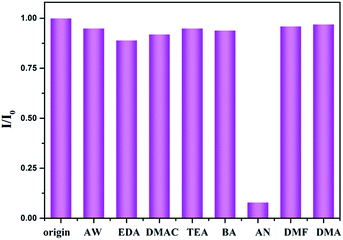 | ||
| Fig. 4 Room-temperature fluorescent intensity of gea-MOF-1 at 373 nm in methanol suspension of gea-MOF-1 with 150 ppm of different organic amines (λex = 290 nm). | ||
In summary, gea-MOF-1 exhibits potential for the selective sensing of NB which can be deployed for explosives, as well as AN. The low detection level, high repeatability and outstanding anti-interference ability make gea-MOF-1 very promising for explosives and organic amines detection, might lead to its applications in biological and environmental systems. These remarkable preliminary results provide us with an impetus to develop RE-based MOFs which find important and multifarious applications.
Acknowledgements
This work was granted financial support from National Natural Science Foundation of China (21271096).Notes and references
- (a) D. Banerjee, Z. C. Hu and J. Li, Dalton Trans., 2014, 43, 10668–10685 RSC; (b) Z. C. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815–5840 RSC; (c) X. J. Zhang, W. J. Wang, Z. J. Hu, G. N. Wang and K. Uvdal, Coord. Chem. Rev., 2015, 284, 206–235 CrossRef CAS; (d) J. H. Cui, Z. Z. Lu, Y. Z. Li, Z. J. Guo and H. G. Zheng, Chem. Commun., 2012, 48, 7967–7969 RSC.
- (a) G. Y. Wang, L. L. Yang, Y. Li, H. Song, W. J. Ruan, Z. Chang and X. H. Bu, Dalton Trans., 2013, 42, 12865–12868 RSC; (b) J. Xiao, Y. Wu, M. Li, B. Y. Liu, X. C. Huang and D. Li, Chem.–Eur. J., 2013, 19, 1891–1895 CrossRef CAS PubMed; (c) A. Lan, K. Li, H. Wu, D. H. Olson, T. J. Emge, W. Ki, M. Hong and J. Li, Angew. Chem., Int. Ed., 2009, 48, 2334–2338 CrossRef CAS PubMed; (d) J. Xiao, Y. Wu, M. Li, B. Y. Liu, X. C. Huang and D. Li, Chem.–Eur. J., 2013, 19, 1891–1895 CrossRef CAS PubMed; (e) S. Pramanik, C. Zheng, X. Zhang, T. J. Emge and J. Li, J. Am. Chem. Soc., 2011, 133, 4153–4155 CrossRef CAS PubMed.
- (a) R. Benigni and L. Passerini, Mutat. Res., Rev. Mutat. Res., 2002, 511, 191–206 CrossRef CAS; (b) P. Vineis and R. Pirastu, Cancer Causes Control, 1997, 8, 346–355 CrossRef CAS PubMed; (c) X. Zhang, X. Liu, R. Lu, H. Zhang and P. Gong, J. Mater. Chem., 2012, 22, 1167–1172 RSC; (d) T. Chu, H. Liu, Y. Yang, H. Wang, Y. Hu, Y. Wang, M. Yu and S. W. Ng, J. Photochem. Photobiol., A, 2014, 294, 38–43 CrossRef CAS.
- (a) K. Hakansson, R. V. Coorey, R. A. Zubarev, V. L. Talrose and P. Hakansson, J. Mass Spectrom., 2000, 35, 337–346 CrossRef CAS PubMed; (b) J. M. Sylvia, J. A. Janni, J. D. Klein and K. M. Spencer, Anal. Chem., 2000, 72, 5834–5840 CrossRef CAS PubMed; (c) S. J. Toal and W. C. Trogler, J. Mater. Chem., 2006, 16, 2871–2883 RSC; (d) Y. L. Hou, H. Xu, R. R. Cheng and B. Zhao, Chem. Commun., 2015, 51, 6769–6772 RSC; (e) Z. L. Wu, J. Dong, W. Y. Ni, B. W. Zhang, J. Z. Cui and B. Zhao, Inorg. Chem., 2015, 54, 5266–5272 CrossRef CAS PubMed.
- (a) H. Xu, F. Liu, Y. Cui, B. Chen and G. Qian, Chem. Commun., 2011, 47, 3153–3155 RSC; (b) M. Guo and Z. M. Sun, J. Mater. Chem., 2012, 22, 15939–15946 RSC; (c) S. S. Nagarkar, B. Joarder, A. K. Chaudhari, S. Mukherjee and S. K. Ghosh, Angew. Chem., Int. Ed., 2013, 52, 2881–2885 CrossRef CAS PubMed; (d) X. Zhou, H. Li, H. Xiao, L. Li, Q. Zhao, T. Yang, J. Zuo and W. Huang, Dalton Trans., 2013, 42, 5718–5723 RSC; (e) F. Y. Yi, J. P. Li, D. Wu and Z. M. Sun, Chem.–Eur. J., 2015, 21, 11475–11482 CrossRef CAS PubMed; (f) S. Dang, T. Wang, F. Y. Yi, Q. H. Liu, W. T. Yang and Z. M. Sun, Chem.–Asian J., 2015, 10, 1703–1709 CrossRef CAS PubMed.
- (a) H. Furukawa, N. Ko, Y. B. Go, N. Aratani, S. B. Choi, E. Choi, A. O. Yazaydin, R. Q. Snurr, M. O'Keeffe, J. Kim and O. M. Yaghi, Science, 2010, 329, 424–428 CrossRef CAS PubMed; (b) M. Eddaoudi, D. F. Sava, J. F. Eubank, K. Adil and V. Guillerm, Chem. Soc. Rev., 2015, 44, 228–249 RSC; (c) Q. L. Zhu and Q. Xu, Chem. Soc. Rev., 2014, 43, 5468–5512 RSC; (d) K. K. Tanabe and S. M. Cohen, Inorg. Chem., 2010, 49, 6766–6774 CrossRef CAS PubMed.
- (a) S. L. Xie, H. F. Wang, Z. H. Liu, R. K. Dai and L. Z. Huang, RSC Adv., 2015, 5, 7121–7124 RSC; (b) J. S. Qin, S. J. Bao, P. Li, W. Xie, D. Y. Du, L. Zhao, Y. Q. Lan and Z. M. Su, Chem.–Asian J., 2014, 9, 749–753 CrossRef CAS PubMed; (c) S. Dang, X. Min, W. T. Yang, F. Y. Yi, H. P. You and Z. M. Sun, Chem.–Eur. J., 2013, 19, 17172–17179 CrossRef CAS PubMed.
- Z. Y. Guo, X. Z. Song, H. P. Lei, H. L. Wang, S. Q. Su, H. Xu, G. D. Qian, H. J. Zhang and B. L. Chen, Chem. Commun., 2015, 51, 376–379 RSC.
- X. M. Cao, N. Wei, L. Liu, L. Li and Z. B. Han, RSC Adv., 2016, 6, 19459–19462 RSC.
- V. Guillerm, Ł. J. Weseliński, Y. Belmabkhout, A. J. Cairns, V. D'Elia, Ł. Wojtas, K. Adil and M. Eddaoudi, Nat. Chem., 2014, 6, 673–680 CAS.
- M. L. Gao, N. Wei and Z. B. Han, RSC Adv., 2016, 6, 60940–60944 RSC.
- (a) S. Pramanik, C. Zheng, X. Zhang, T. J. Emge and J. Li, J. Am. Chem. Soc., 2011, 133, 4153–4155 CrossRef CAS PubMed; (b) B. Liu, W. P. Wu, L. Hou and Y. Y. Wang, Chem. Commun., 2014, 50, 8731–8734 RSC; (c) F. Y. Yi, W. T. Yang and Z. M. Sun, J. Mater. Chem., 2012, 22, 23201–23209 RSC.
- (a) J. Z. Wang, W. Sun, S. Y. Chang, H. T. Liu, G. N. Zhang, Y. Q. Wang and Z. L. Liu, RSC Adv., 2015, 5, 48574–48579 RSC; (b) J. Song, X. Gao, Z. N. Wang, C. R. Li, Q. Xu, F. Y. Bai, Z. F. Shi and Y. H. Xing, Inorg. Chem., 2015, 54, 9046–9059 CrossRef CAS PubMed; (c) S. L. Xie, H. F. Wang, Z. H. Liu, R. Dai and L. Z. Huang, RSC Adv., 2015, 5, 7160–7172 RSC; (d) S. N. Zhao, X. Z. Song, M. Zhu, X. Meng, L. L. Wu, S. Y. Song, C. Wang and H. J. Zhang, RSC Adv., 2015, 5, 93–98 RSC; (e) X. L. Hu, F. H. Liu, C. Qin, K. Z. Shao and Z. M. Su, Dalton Trans., 2015, 44, 7822–7827 RSC; (f) F. H. Liu, C. Qin, Y. Ding, H. Wu, K. Z. Shao and Z. M. Su, Dalton Trans., 2015, 44, 1754–1760 RSC; (g) W. Xie, S. R. Zhang, D. Y. Du, J. S. Qin, S. J. Bao, J. Li, Z. M. Su, W. W. He, Q. Fu and Y. Q. Lan, Inorg. Chem., 2015, 54, 3290–3296 CrossRef CAS PubMed; (h) M. M. Chen, X. Zhou, H. X. Li, X. X. Yang and J. P. Lang, Cryst. Growth Des., 2015, 15, 2753–2760 CrossRef CAS; (i) X. L. Hu, C. Qin, X. L. Wang, K. Z. Shao and Z. M. Su, New J. Chem., 2015, 39, 7858–7862 RSC; (j) X. L. Hu, C. Qin, L. Zhao, F. H. Liu, K. Z. Shao and Z. M. Su, RSC Adv., 2015, 5, 49606–49613 RSC; (k) X. M. Cao, N. Wei, L. Liu, L. Li and Z. B. Han, RSC Adv., 2016, 6, 19459–19462 RSC.
- (a) S. S. Nagarkar, B. Joarder, A. K. Chaudhari, S. Mukherjee and S. K. Ghosh, Angew. Chem., Int. Ed., 2013, 52, 2881–2885 CrossRef CAS PubMed; (b) X. D. Zhu, Y. Li, W. X. Zhou, R. M. Liu, Y. J. Ding, J. Lü and D. M. Proserpio, CrystEngComm, 2016, 18, 4530–4537 RSC.
- (a) D. Tian, Y. Li, R. Y. Chen, Z. Chang, G. Y. Wang and X. H. Bu, J. Mater. Chem. A, 2014, 2, 1465–1470 RSC; (b) K. Xiang, Y. M Li, C. H. Xu and S. H. Li, J. Mater. Chem. C, 2016, 4, 5578–5583 RSC; (c) J. Q. Liu, J. Wu, F. M. Li, W. C. Liu, B. H. Li, J. Wang, Q. L. Li, R. Yadav and A. Kumar, RSC Adv., 2016, 6, 31161–31166 RSC.
- (a) S. M. Hu, H. L. Niu, L. G. Qiu, Y. P. Yuan, X. Jiang, An. J. Xie, Y. H. Shen and J. F. Zhu, Inorg. Chem. Commun., 2012, 17, 147–150 CrossRef CAS; (b) S. S. Zhao, J. Yang, Y. Y. Liu and J. F. Ma, Inorg. Chem., 2016, 55, 2261–2273 CrossRef CAS PubMed; (c) H. Zhang, J. Yang, Y. Y. Liu, S. Y. Song and J. F. Ma, Cryst. Growth Des., 2016, 16, 3244–3255 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, PXRD, luminescence spectra. See DOI: 10.1039/c6ra19133a |
| This journal is © The Royal Society of Chemistry 2016 |