Self-assembly and structure of flagellin–polyelectrolyte composite layers: polyelectrolyte induced flagellar filament formation during the alternating deposition process†
Abstract
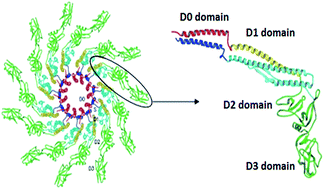
The simple and cost-effective bottom-up fabrication of complex functionalized nanostructures is extensively researched today. Here, the alternating deposition of the negatively charged protein flagellin and a positively charged polyelectrolyte is studied. The multilayer buildup was followed in situ using Optical Waveguide Lightmode Spectroscopy (OWLS) revealing the deposited surface mass density in real time during the alternating deposition process. The nanostructure of the assembled films was investigated by Atomic Force Microscopy (AFM) measurements. When flagellin was applied in its natural filamentous form no distinct multilayer buildup was observed, the filaments assembled mainly into bundles. In contrast, when thermally treated filament solution or pure flagellin monomer solution was used a systematic linearly growing buildup was seen, and thick, relatively smooth films were fabricated. AFM investigations revealed that the polycation induced assembly of flagellin monomers into nanofilaments during the deposition process. Both the filament formation and the multilayer buildup were completely absent when a truncated flagellin variant – missing the disordered terminal regions – was applied. Since these regions are necessary for filament formation, we conclude that the linearly growing nature of the layer is a clear consequence of filament formation. Therefore, this study first reveals a new type of linearly growing polyelectrolyte multilayer buildup mechanism, when one of the components induces the self-assembly of the oppositely charged component, creating a complex, stable and smooth filamentous nanostructured coating. These composite films can find diverse applications in nanotechnology and in biomedical sciences since the variable D3 domain of flagellin subunits can be easily modified to express enzymatic, fluorescent or molecular binding properties on the surfaces of the filaments.

 Please wait while we load your content...
Please wait while we load your content...