High shielding effectiveness of multilayer graphene oxide aerogel film/polymer composites
Abstract
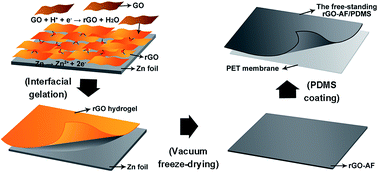
In this work, an efficient and simple method is proposed to fabricate lightweight and flexible composites composed of a reduced graphene oxide aerogel film (rGO-AF) and a polydimethylsiloxane (PDMS) matrix. Their morphology, electromagnetic interference (EMI) shielding effectiveness (SE), and corresponding mechanisms are studied. The rGO-AF was obtained by a process with combinations of zinc reduction, freeze-drying, peeling and stacking. Then, single and multilayer rGO-AF/PDMS composites were fabricated by liquid PDMS infiltrating and curing. The shielding effectiveness of the composites is found to depend on the gelation time. The prepared five-layer composite with a density of 0.122 g cm−3 and a thickness of 1.547 mm, has an SE value of 53 dB at X band (8.2–12.4 GHz), and a specific SE of up to 434 dB cm3 g−1. Based on the measurements on absorption and reflection shielding effectiveness, the high electromagnetic irradiation (EMI) shielding effectiveness of rGO-AF/PDMS composites is attributed to the absorption dominated shielding attenuation.

 Please wait while we load your content...
Please wait while we load your content...