Synergistic effects of filler-migration and moisture on the surface structure of polyamide 6 composites under an electric field†
Abstract
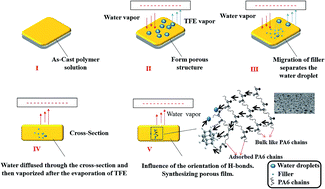
Polyamide 6 (PA6) and PA6 composites with 2 wt% of nanofillers (aminopropyl isobutyl POSS (AB-POSS) or polymer grade montmorillonite (PGN)) were synthesized by electric assisted phase inversion at different moistures. The breath figure phenomenon occurred spontaneously although the moisture of the atmosphere just reached 55%, owing to the fast evaporation rate of 2,2,2-trifluoro ethanol under the electric field. The results of X-ray diffraction indicated that the high moisture disturbed the original arrangement of H-bonded planes in the PA6, which affected the crystalline structure of PA6 and the dispersion of PGN. The PGN and AB-POSS had different migration behaviors under the electric field. This migration behavior and the moisture showed synergistic influences on the surface structure of the PA6 composites. Water contact angle measurements showed that the surface of PA6 composites was able to switch from hydrophobic to hydrophilic (i.e., 58.7–125.8°) owing to the synergistic effects of filler-migration and moisture. The proposed formation mechanism of the PA6 composites under the electric was discussed.

 Please wait while we load your content...
Please wait while we load your content...