Aluminum derivative peroxides in the (t-BuO)3Al–2t-BuOOH catalytic system as a source of electron-excited dioxygen: a quantum chemical study on a model†
Abstract
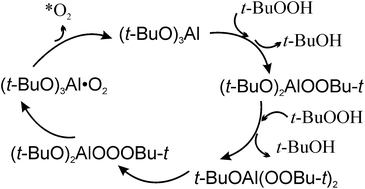
The quantum chemical study of the MeOOH/(MeO)3Al model system has been carried out in order to predict the mechanism of the catalytic decomposition of t-BuOOH under mild conditions for the t-BuOOH/(t-BuO)3Al system being a powerful synthetic tool for selective oxidation. To elucidate the chemical excitation of O2 eliminated in the catalytic reaction and to predict the electronic state of O2, the topology of the potential energy surface (PES), the structures of intermediates and transition states, the activation and reaction energies were obtained at the B3LYP/cc-pVTZ theory level. It was shown that the peroxide, (MeO)2AlOOMe, corresponding to the experimentally obtained (t-BuO)2AlOOBu-t, is formed in the first step of the reaction. After that, in the main pathway, the aluminum-containing peroxide reacts with the second MeOOH molecule through the nucleophilic substitution of the second methoxy group forming the MeOAl(OOMe)2 diperoxide. The diperoxide rearranges to aluminum-containing ozonide MeOAlOOOMe. The ozonide isomerizes in the mononuclear-metal dioxygen intermediate (MeO)3Al·O2. The latter decomposes through the adiabatic ((MeO)3Al + O2(b1Σ+g)) and non-adiabatic ((MeO)3Al + O2(X3Σ−g)) pathways, which corresponds to experimental data about the incomplete conversion of O2 to O2(b1Σ+g). The generation of O2(b1Σ+g) was revealed by the analysis of the energy diagram calculated with the CCSD(T), CCSDT(Q), and CASSCF methods. It was suggested that the η1-(MeO)3Al·O2 and, thus, (t-BuO)3Al·O2 complexes are new sources of O2(b1Σ+g).

 Please wait while we load your content...
Please wait while we load your content...