Antioxidative activity of methyl amygdalinate from the seeds of Prunus persica and neuroprotective effects on Aβ1–42-induced neurodegeneration models†
Xu Zhaoa,
Zhengzheng Liaob,
Yu Qib,
Xu Shenb,
Kaishun Bic and
Ying Jia*a
aSchool of Functional Food and Wine, Shenyang Pharmaceutical University, Wenhua Road 103, Shenyang 110016, China. E-mail: jiayingsyphu@126.com; Tel: +86 24 23986933
bSchool of Traditional Chinese Materia Medica, Shenyang Pharmaceutical University, Wenhua Road 103, Shenyang 110016, China
cSchool of Pharmacy, Shenyang Pharmaceutical University, Wenhua Road 103, Shenyang 110016, China
First published on 27th September 2016
Abstract
Prunus persica has been frequently used as a functional and medicinal food in China and other Asian countries. Aromatic glucosides isolated from the dried seeds of P. persica have been reported to possess antioxidant activity. The aim of this study was to investigate methyl amygdalinate (MAM), an active aromatic glucoside from P. persica with antioxidative and neuroprotective activities, in an Aβ1–42-induced mouse model of Alzheimer's disease. Neuroprotective effects of MAM (10, 100 μg kg−1) were estimated through the spontaneous locomotor test and the Morris water maze test. Antioxidative effects of MAM were evaluated by measurement of SOD and MDA levels in the hippocampus and cerebral cortex of model mice. In addition, the expressions of BDNF (neurotrophic factor), JKN/p38 (both contribute to MAPKs signaling pathways) in mouse brain were measured. Histopathological examination was used to represent the potential mechanisms. It was found that intracerebroventricular (i.c.v.) administration of MAM to Aβ1–42-induced mice significantly increased the swimming time in the target quadrant in the Morris water maze test. It also noticeably decreased SOD activity, and restored MDA level both in the hippocampus and cerebral cortex in mice. Furthermore, MAM could change the expressions of BDNF, and inhibit the expression of JKN/p38 in mouse brain. Results of histopathological examination also indicated that MAM markedly ameliorated neurodegeneration in the hippocampus in mice. In summary, MAM might protect against cognitive deficits and neurodegeneration by releasing the damage of oxidative stress and inhibiting the MAPKs inflammatory signaling pathways.
Introduction
Alzheimer's disease (AD) is an important age-related neurodegenerative disorder which interrupts various functions of the brain like memory, intelligence, judgment and learning abilities.1 AD is characterized by the over-production of amyloid-beta (Aβ) and intracellular phospho-tau-positive neurofibrillary tangles.2 Overproduction or lack of clearance of Aβ leads to an increased aggregation of Aβ which could simulate the pathogenesis of AD.3 In the brain of AD patients, Aβ peptide prominently increases, producing a much larger amyloid precursor protein (APP), and leads to synaptic integrity loss, degeneration, neurite dystrophy, microglia and astrocyte activation and neuronal apoptosis.4,5 Moreover, the pathophysiology of AD has been evidenced to involvement in oxidative stress and is accompanied by the activation of the presence of glic-mediated inflammatory response as well.6,7 Thus, any compound which could furnish beneficial effects including the enhancement of antiapoptotic signaling, the inhibition of the apoptotic pathway, or decreasing the inflammation process may potentially serve as a therapeutic for AD.8 Recently, natural products have served as the source for a large fraction of drugs and active compounds.Peach (Prunus persica (L.) Batsch), is a very popular fruit all over the world. Persicae Semen (namely Taoren in Chinese), the dried seed of P. persica, is a commonly used functional and medicine food in China and other Asian countries. It has been used for centuries for the treatment of degenerative disorders, such as hypermenorrhea, dysmenorrhea, leiomyoma and infertility,9–12 and additionally showed a strong effects of anti-tumour and anti-Oketsu syndrome (stagnation of blood circulations).13,14 The chemical constituents of Persicae Semen include the cyanogenic glucosides, amygdalin and prunasin as the major components, along with the glycerides and sterols.15,16 Based on the published paper, aromatic glucosides from Persicae Semen exhibited inhibition of malondialdehyde (MDA) production against Fe2+–cysteine induced rat liver microsomal lipid peroxidation, indicating preliminary antioxidative activity in vitro.17 In the present study, we investigated the effect of methyl amygdalinate (MAM), an active aromatic glucoside compound isolated from Persicae Semen, on Aβ1–42-induced neurodegeneration with cognitive impairment in the mouse of AD models. Biochemical analyses and histopathological examination were used to represent the potential mechanisms.
Materials and methods
Chemicals, reagents and materials
The seeds of Prunus persica were purchased from Tongrentang Pharmacy (Shenyang, China), and identified by Professor Ying Jia, expert of traditional Chinese medicine appraisal (Shenyang Pharmaceutical University). Aβ1–42 peptide and donepezil were obtained from Sigma (St. Louis, MO, USA). Dulbecco's modified eagle's medium (DMEM), dimethyl sulphoxide (DMSO), fetal bovine serum (FBS) were purchased from Hyclone (Logan, UT, USA). The assay kits of superoxide dismutase (SOD), malondialdehyde (MDA), brain derived neurotrophic factor (BNDF), c-Jun N-terminal kinase (JNK) and p38 were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).Male, 10 week-old, Kunming mice weighing 25–30 g were purchased from the Central Animal House of Shenyang Pharmaceutical University (Shenyang, China). Mice were housed in groups of 6 per cage, with ad libitum access to water and food, and maintained in constant temperature (23 ± 1 °C) and humidity (55 ± 5%). The study was conducted in accordance with the Guidelines for Animal Experimentation and the protocol was approved by the Animal Ethics Committee of Shenyang Pharmaceutical University.
Isolation and identification of methyl amygdalinate
Dried Persicae Semen (18 kg) was extracted with 40 L ligroin (×2) under reflux for 2 h. The residues were extracted with 80 L 70% ethanol (×3) under reflux for 2 h. The extracts were combined and concentrated to a small volume (∼10 L), and successively extracted with acetoacetate (10 L × 3) and n-butyl alcohol (10 L × 3). Evaporating of the solvents was done with a rotary evaporator under vacuum, and which provided 719.7 g of n-butanolic extract. Part of the extract (100 g) was subjected to column chromatography over D-101 macroporous resin (D-101) and eluted with gradient ethanol–water solvent system resulted in four fractions. MAM was purified from Fraction One (30% ethanol, v/v) using a Shimadzu semi-preparative HPLC system. 1H-NMR, 13C-NMR spectra of MAM were acquired on Bruker ARX-300 NMR spectrometer, and HSQC, HMBC spectra were acquired on Bruker Avance-600 NMR spectrometers. Bruker MicrOTOF-Q mass spectrometer was used for HRESIMS analysis. Optical rotation was measured with a Jasco P-2000 polarimeter, HPLC was performed on an Agilent 1260 instrument equipped with a detector, using a RD-LC001 C18 column (250 × 15 mm, 4.6 μm). Structure elucidation was based on interpretation of NMR, HPLC, mass spectroscopic and optical rotation data.Neuroprotective effects of MAM in vitro
The preliminary protective effects of MAM against Aβ1–42-induced damage in vitro were tested with primary mouse neuron cells. The cells were grown in DMEM/F12 supplemented with 10% FBS. Cells were seeded into 96-well plates (1 × 106 cells per well) pre-coated with 0.01% poly-L-lysine, and cultured in Neurobasal medium supplemented with 2% B27 and 100 U ml−1 penicillin, 100 μg ml−1 streptomycin at 37 °C in 10% CO2. After 24 h subculture, the medium was replaced with fresh medium and the attached cells were exposed to 10 μM Aβ1–42 in the presence or absence of MAM (10 and 100 μM), and donepezil (10 μM) was used as the positive drug. After culture for 48 h, cell viability was determined using the MTT assay.18,19Neuroprotective effects of MAM in Aβ1–42-induced dementia mice
Biochemical analysis
On the day after behavioral tests, all the mice were sacrificed by cervical dislocation and the brain was immediately removed. The hippocampus and cerebral cortex of eight mice in each group were dissected out, and stored at −80 °C until the biochemical studies. The entire brain of the other four mice in each group were fixed in 10% formalin at 4 °C and then embedded in paraffin for histopathological analysis.Before detection, each part of the brain tissue was rapidly homogenized in ice-cold saline and centrifuged at 3500 rpm at 4 °C for 15 min. The supernatants of the hippocampus and cerebral cortex were used to measure the activities of antioxidative enzymes including SOD, MDA levels, and the protein expressions of BDNF, JNK and p38 by ELISA according to the manufacturer's directions.
Histopathological examination
The entire brain was postfixed in 4% paraformaldehyde (PFA) solution for 48 h, then transferred to 30% sucrose in 0.1 mol L−1 PBS (pH 7.4) for atleast 16 h. The hippocampuses were then kept in the final sucrose solution until sectioning. Serial sections of 10 μm thickness were cut and stained with haematoxylin and eosin.Statistical analysis
All statistical analyses were performed with SPSS software, version 19.0. Data are expressed as the mean ± S.E.M. (n = number of experiments). Results were obtained by analysis of variance (ANOVA), followed by Tukey's multiple comparison test, with p-values < 0.05 considered to be significant.Results and discussion
Structure elucidation of the unknown compound
The unknown compound was obtained as a white, amorphous powder, HRESIMS gave a quasimolecular ion peak at m/z 513.1575 [M + Na]+, corresponding to the molecular formula C21H30O13. [α]20D = −112.6° (c 0.2, CH3OH) for the aglycone after hydrolyzing with acid. The 1H and 13C NMR data (Table 1) showed the presence of a benzyl group [δ: 5.48 (1H, s, H-7), 78.8 (C-7)], a methoxy group [δ: 3.71 (3H, s, –OCH3), 53.3 (–OCH3)], and an ester carbonyl [δ: 173.3 (C-8)]. The HMBC correlations at H–OCH3 (δ: 3.71)/C-8 (δ: 173.3), H-1′′ (δ: 4.45)/C-6′ (δ: 104.8) and H-1′ (δ: 4.28)/C-7 (δ: 78.8) suggested the presence of a methyl ester group and revealed that the sugar chain was glucopyranosyl-(1–6)-glucopyranoside and linked to the benzyl group. Spectra of 1H, 13C, HSQC, HMBC and HPLC chromatogram of the unknown compound were shown as ESI Fig. 1–5.† The structure was elucidated as (R)-phenylacetic acid methyl ester α-[(6-O-β-glucopyranosyl-β-D-glucopyranosyl)oxy], named (R)-methyl amygdalinate (Fig. 1).| Position | δH | δC | HMBC |
|---|---|---|---|
| 1 | 136.5 | ||
| 2,6 | 7.54 (2H, m) | 128.9 | C-4, C-7 |
| 3,5 | 7.38 (2H, m) | 129.9 | C-1, C-2, C-6 |
| 4 | 7.40 (1H, m) | 130.2 | |
| 7 | 5.48 (1H, s) | 78.8 | C-1, C-2, C-8, C-1′ |
| 8 | 173.3 | ||
| –OCH3 | 3.71 (3H, s) | 53.3 | C-8 |
| Glc-1′ | 4.28 (1H, d, J = 7.6 Hz) | 101.1 | C-7 |
| 2′ | 75.2 | ||
| 3′ | 78.8 | ||
| 4′ | 71.5 | ||
| 5′ | 77.5 | ||
| 6′ | 69.2 | ||
| Glc-1′′ | 4.45 (1H, d, J = 7.6 Hz) | 104.8 | C-6′ |
| 2′′ | 74.7 | ||
| 3′′ | 7.9 | ||
| 4′′ | 71.3 | ||
| 5′′ | 77.2 | ||
| 6′′ | 62.7 |
Effects of MAM on cell viability of Aβ1–42-induced primary mouse neuronal cells
We firstly investigated the toxicity and neuroprotective effects in vitro by determination of cell viability with MTT assay. According to the results, Aβ1–42 group generated a dramatic decrease in the OD value compared with the control group (p < 0.01). However, MAM (100 and 10 μM) significantly restored the decrease in cell viability (p < 0.01, p < 0.05, respectively). The cell viability reached up to 78.31% and 72.51%, when cells were treated with MAM, respectively (Fig. 2).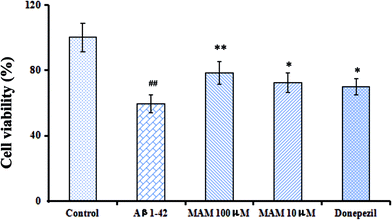 | ||
| Fig. 2 Effects of MAM on cell viability of primary mouse neuronal cells induced by Aβ1–42. #p < 0.05, ##p < 0.01 compared with the control group; *p < 0.05, **p < 0.01 compared with Aβ1–42 group. | ||
Effect of MAM on the performance of Aβ1–42-induced mice in the locomotor activity test
The influence of the surgery and i.c.v. administration on the mice was investigated by the locomotor activity test. The results of nearby test showed that the surgery and i.c.v. administration had no bearing on the locomotion activity of mice in each group (Fig. 3). There were no significant differences in the numbers of locomotion activity between Aβ1–42 group and control group, suggesting that the modeling method and drug treatment did not impact on their locomotor activity.Effect of MAM on the performance of Aβ1–42-induced mice in the Morris water maze test
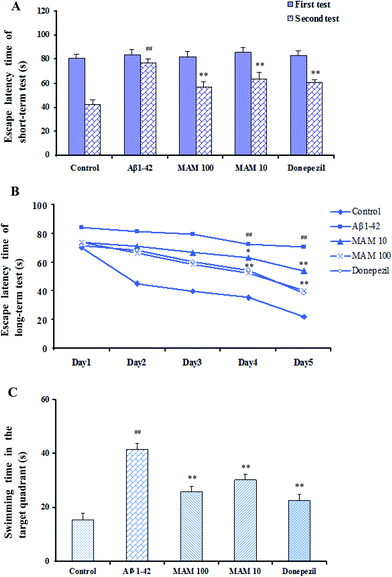
The effect of MAM on short-term was investigated on the first day by using the Morris water maze test. The escape latency time Aβ1–42 treated mice spent was longer than that of control group mice, and was significantly reversed by MAM (10 and 100 μg kg−1), respectively (both p < 0.01, Fig. 4A). To reveal the effects of MAM on improving the impairment of spatial learning and memory in Aβ1–42-induced mouse model, long-term test was performed. The escape latency time to find a platform in the water maze was used as a parameter for evaluating the performance of the tested mice. The Aβ1–42 treated group showed significant delay on the escape latency time compared with the control group on day 4 and 5, whereas both dose levels of MAM significantly decreased the escape latency time to find the platform on day 4 (p < 0.05, p < 0.01) and day 5 (p < 0.01, p < 0.05, Fig. 4B). On the probe trial day, mice were expected to spend the majority of the trial searching for the platform in the target quadrant to detect the memory ability. MAM treatment increased the swimming time in the target quadrant after the platform was removed (p < 0.01, Fig. 4C).Effects of MAM on SOD and MDA levels in the hippocampus and cerebral cortex of the Aβ1–42-induced mice
We evaluated SOD and MDA levels in the hippocampus and cerebral cortex to elucidate whether Aβ1–42 injection makes any change in the antioxidant status within the brain or this change is reversible by the treatment of MAM. Compared with the control group, Aβ1–42 treatment generated a dramatic decrease in SOD activity as well as a significant increase in MDA production both in the hippocampus and cerebral cortex (p < 0.01, respectively). However, both dose levels of MAM significantly restored SOD activity in the hippocampus and cerebral cortex (p < 0.01, respectively, Fig. 5A). The increase in MDA production was markedly attenuated by the treatment with MAM (100 μg kg−1) in the hippocampus and cerebral cortex (p < 0.05, respectively, Fig. 5B).Effects of MAM on the expression of BDNF in the hippocampus and cerebral cortex of the Aβ1–42-induced mice
In order to evaluate the role of BDNF in the neurotrophic factors of Aβ1–42 injected mice, the protein expression of BDNF was determined in the hippocampus and cerebral cortex of mice. As showed in Fig. 6, BDNF levels in model group were lower than that of control group (p < 0.01), and the decrease of BDNF were noticeably inhibited by MAM (100 and 10 μg kg−1) in both hippocampus and cerebral cortex (p < 0.01, p < 0.05, respectively), indicating significant neuroprotective effect.Effects of MAM on MAPKs inflammatory signaling pathways in the hippocampus and cerebral cortex of the Aβ1–42-induced mice
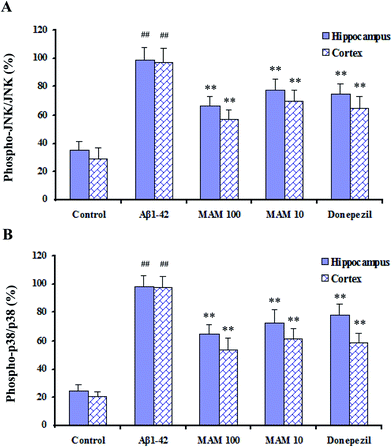
The effects that MAM exerted on the activation of MAPKs inflammatory signaling pathways were determined in Aβ1–42-induced mice. It was found that MAM (10 and 100 μg kg−1) significantly inhibited the phosphorylation of JNK and p38, which both contributed to MAPKs signaling pathways, while their non-phosphorylated forms remained unchanged in hippocampus and cerebral cortex in the Aβ1–42-induced mice (Fig. 7A and B).Effect of MAM on histopathological changes in the hippocampus of mice
HE staining was performed to detect the neuronal integrity and orderliness in the hippocampus. The neuronal layers in the CA1 region of the hippocampus have shown rarefaction, disordered, pronounced shrinkage nuclei and swollen neuronal bodies in the model group (Fig. 8B) compared with the control group (Fig. 8A). MAM 10 μg kg−1 treated group (Fig. 8C), and 100 μg kg−1 treated group (Fig. 8D) significantly inhibited the histopathological damage, as those pictures shown the nucleoli were clearly visible, and there was no edema cell.Discussion
Alzheimer's disease is probably caused by complex interactions, and the pathogenic mechanisms underlying AD include impaired cholinergic function, increased oxidative stress, overproduction of Aβ peptides, an increase of neuronal apoptosis, a dysregulation of neuroplasticity-related molecules and expression of inflammatory mediators and so on.23 However, compelling evidences suggested that the overproduction of Aβ peptides, especially Aβ1–42, oxidative stress and apoptosis induced by Aβ play a critical role in the progressive of AD. Mice intracerebroventricular injected with Aβ can mimic some cognitive deficits in AD, which is considered to be a reliable model. In this study, we established the AD models induced by Aβ1–42 to investigated some important biochemical parameters about oxidative stress, inflammation and cell damage.To reveal the neuroprotective effects of MAM on the Aβ1–42-induced models, we firstly evaluated the toxicity of MAM by determination of cell viability of the primary mouse neuronal cells. Overall, pretreatment with MAM markedly inhibited cell death induced by Aβ1–42 exposure as determined by cell viability assay with no toxicity. Furthermore, the animal-relevant behavioral studies indicated that MAM significantly alleviated cognitive deficits validated by the behavioral tests in Aβ1–42-induced mouse models of Alzheimer's disease.
The improvement of the activities of SOD, as well as the decrease on the levels of MDA indicated that MAM could restore the biochemical pathological changes. SOD is an important enzyme in the oxidation system, which protected cellular against damage caused by oxygen-derived free radicals.24,25 MDA is one of the primary products of lipid peroxidation and it directly damages cell membranes.26 In our study, mice in the control group became amnesia, and the biochemical parameters of oxidative stress and cell damage were improved by MAM.
BDNF is the most prevalent neurotrophic factor in adult brains, which plays a critical role in cognition, learning and memory, facilitates neurogenesis and synapse formation, also reduces oxidative stress and cell death.27 BDNF is normally produced in the cortex and transported to the hippocampus anterogradely to support the physiological functions of memory circuitry.28 Decline in BDNF levels have been implicated in early stages of AD pathogenesis, leading to dementia.29 The decrease of BDNF in Aβ1–42-induced mice were noticeable inhibited by MAM in our test, indicating significant neuroprotective effect.
Moreover, the effects of MAM exerted on the activation of MAPK family in mice was determined. The MAPK family takes part in the induction of proinflammatory cytokines and chemokines, tightly associated with oxidative stress and inflammation.30 It was found that MAM significantly decreased the expression of JNK and p38 signaling pathways, by inhibiting the phosphorylation of JNK and p38, indicating significant anti-inflammatory effects.
The results of animal-relevant study suggested that MAM might protect against cognitive deficits and neurodegeneration by releasing the damage of oxidative stress, showing neuroprotective, and anti-inflammatory effects on the Aβ1–42-induced mice. Furthermore, the results of histopathological in the hippocampus proved that MAM noticeably ameliorated the pathological changes induced by Aβ1–42 in mice.
Conclusion
The present study demonstrated that methyl amygdalinate, an active aromatic glucoside from Persicae Semen, reversed alterations in cognitive behavioral, biochemical and histopathological changes induced by Aβ1–42 in mice. These beneficial effects may be partially attributed to releasing the damage of oxidative stress, showing neuroprotective effect, and inhibiting the MAPKs inflammatory signaling pathways. Our findings suggested that methyl amygdalinate and other aromatic glucosides from the seed of Prunus persica might be worthwhile functional and medicine food in the treatment of cognitive and behavioral deficits.Ethical statement
This study was conducted in accordance with the Guidelines for Animal Experimentation and the protocol was approved by the Animal Ethics Committee of Shenyang Pharmaceutical University. All the cells and tissues of the animals were authorized to scientific purpose.Conflict of interest
The authors have declared that there is no conflict of interest.Abbreviations
| MAM | Methyl amygdalinate |
| SOD | Superoxide dismutase |
| MDA | Malondialdehyde |
| AD | Alzheimer's disease |
| BNDF | Brain derived neurotrophic factor |
| JNK | Jun N-terminal kinase |
| DMEM | Dulbecco's modified eagle's medium |
| DMSO | Dimethyl sulphoxide |
| FBS | Fetal bovine serum |
Acknowledgements
This research was supported by National Natural Science Foundation of China (No. 81573580 and No. 81403065) and Education Department of Liaoning Province (No. L2015533).References
- M. Jay and D. O. Ellis, J. Am. Osteopath. Assoc., 2005, 3, 145–158 Search PubMed.
- J. J. Palop and L. Mucke, Nat. Neurosci., 2010, 13, 812–818 CrossRef CAS PubMed.
- J. Hardy and D. J. Selkoe, Science, 2002, 297, 353–356 CrossRef CAS PubMed.
- S. Kar, S. P. Slowikowski, D. Westaway and H. T. Mount, J. Psychiatr. Neurosci., 2004, 29, 427–441 Search PubMed.
- L. Gu and Z. Guo, J. Neurochem., 2013, 126, 305–311 CrossRef CAS PubMed.
- D. A. Butterfield and E. R. Stadtman, Adv. Cell Aging Gerontol., 1997, 2, 161–191 CAS.
- H. Capiralla, V. Vingtdeux, H. T. Zhao, R. Sankowski, Y. Al-Abed, P. Davies and P. Marambaud, J. Neurochem., 2012, 120, 461–472 CrossRef CAS PubMed.
- P. Luciani, C. Deledda, F. Rosati, S. Benvenuti, I. Cellai, F. Dichiara, M. Morello, G. B. Vannelli, G. Danza, M. Serio and A. Peri, Endocrinology, 2008, 149, 4256–4266 CrossRef CAS PubMed.
- S. Sakamoto, H. Kudo, T. Kawasaki, K. Kuwa, N. Kasahara, S. Sassa and R. Okamoto, J. Ethnopharmacol., 1988, 23, 151–158 CrossRef CAS PubMed.
- S. Sakamoto, H. Yoshino, Y. Shirahata, K. Shimodairo and R. Okamoto, Am. J. Chin. Med., 1992, 20, 313–317 CrossRef CAS PubMed.
- D. Wang, Z. Wang and C. Yu, J. Tradit. Chin. Med., 1998, 18, 7–11 CAS.
- R. Y. Ge, C. H. Zhou and Y. C. She, J. Tradit. Chin. Med., 1983, 3, 23–26 CAS.
- T. Kosuge, H. Ishida and M. Ishii, Chem. Pharm. Bull., 1985, 33, 1496–1498 CrossRef CAS PubMed.
- T. Okuyama, M. Matsuda, N. Kishi, S. N. Lee, M. Baba, Y. Okda and H. Nishino, Nat. Med., 1995, 49, 261–265 Search PubMed.
- L. Y. He and B. M. Li, Biomed. Chromatogr., 1988, 2, 271–273 CrossRef CAS PubMed.
- T. Isoza, Y. Matano, K. Yamamoto, N. Kosaka and T. Tani, J. Chromatogr. A, 2002, 923, 249–254 CrossRef.
- X. Y. Chen, H. Q. Wang, T. Zhang, C. Liu, J. Kang, R. Y. Chen and D. Q. Yu, J. Nat. Prod., 2013, 76, 1528–1534 CrossRef CAS PubMed.
- X. Y. Song, J. F. Hu, M. N. Sun, Z. P. Li, D. H. Wu, H. J. Ji, Y. H. Yuan, Z. X. Zhu, N. Han, G. Liu and N. H. Chen, Neuroscience, 2013, 242, 28–38 CrossRef CAS PubMed.
- X. Zhao, C. M. Liu, M. J. Xu, X. L. Li, K. S. Bi and Y. Jia, PLoS One, 2016, 11, e015277220 Search PubMed.
- G. Paxinos and K. B. Franklin, The Mouse Brain in Stereotaxic Coordinates, Academic Press, San Diego, 2nd edn, 2001 Search PubMed.
- X. L. Li, X. Zhao, X. Xu, X. Mao, Z. Liu, H. Li, L. Guo, K. S. Bi and Y. Jia, Physiol. Behav., 2014, 132, 10–16 CrossRef CAS PubMed.
- R. Morris, J. Neurosci. Methods, 1984, 11, 47–60 CrossRef CAS PubMed.
- R. S. Shah, H. G. Lee, W. Z. Xiong, G. Perry, M. A. Smith and R. J. Castellani, Biomed. Pharmacother., 2008, 62, 199–207 CrossRef CAS PubMed.
- A. Mugge, J. Elwell, T. E. Peterson and D. G. Harrison, Am. J. Physiol.: Cell Physiol., 1991, 260, 219–225 Search PubMed.
- Y. Gilgun-Sherki, Z. Rosenbaum, E. Melamed and D. Offen, Pharmacol. Rev., 2002, 54, 271–284 CrossRef CAS PubMed.
- T. F. Slater, Methods Enzymol., 1984, 105, 283–293 CAS.
- T. Leyhe, G. W. Eschweiler, E. Stransky, T. Gasser, P. Annas, H. Basun and C. Laske, J. Alzheimer's Dis., 2009, 16, 649–656 CAS.
- C. A. Altar, N. Cai, T. Bliven, M. Juhasz, J. M. Conner, A. L. Acheson, R. M. Lindsay and S. J. Wiegand, Nature, 1997, 389, 856–860 CrossRef CAS PubMed.
- M. G. Murer, Q. Yan and R. Raisman-Vozari, Prog. Neurobiol., 2001, 63, 71–124 CrossRef CAS PubMed.
- M. Ramin, P. Azizi, F. Motamedi, A. Haghparast and F. Khodagholi, Behav. Brain Res., 2011, 217, 424–431 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra18913j |
| This journal is © The Royal Society of Chemistry 2016 |