Novel synthesis of an iron oxalate capped iron oxide nanomaterial: a unique soil conditioner and slow release eco-friendly source of iron sustenance in plants†
Abstract
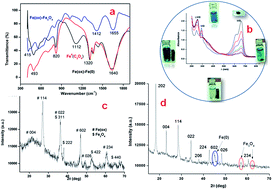
Iron (Fe) is a vital plant-derived micronutrient in the human diet. Fe availability in soil largely depends on the pH and leaching behaviour of the soil. Although common salts (FeSO4) and chelates (EDTA) of Fe ensure high availability of the nutrient, they often interfere with P availability in the soil. Considering such disadvantages of the well-known Fe sources, we attempted to evolve efficient Fe3O4 nanomaterials that are independent of soil reaction (i.e. pH) and do not prevent P solubility in soil. The present investigation resulted in a novel, green and an easy pathway of large-scale synthesis of orthorhombic Fe–oxalate capped-Fe-oxide (Fe3O4) (OCIO) nanomaterial with a prolific agricultural applicability. This nanomaterial did not affect the growth of beneficial soil bacteria and had no phytotoxic effects on seed germination. The Fe release profile from the OCIO was uniform at different pH (4 to 9) conditions due to its exceptional H+ ion scavenging quality. Significantly higher P availability was recorded in aqueous and soil media treated with OCIO as compared to FeSO4 and Fe–EDTA. Additionally, application of OCIO@10–20 mg kg−1 considerably increased organic C, N, P, and enzyme activity in soil. Furthermore, the OCIO dramatically recovered Fe deficiency, maintained steady P availability, and stabilized pH in poorly fertile soil which promoted healthy growth and productivity of tomato.

 Please wait while we load your content...
Please wait while we load your content...