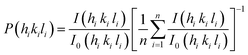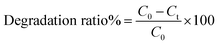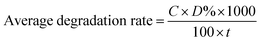DOI:
10.1039/C6RA18838A
(Paper)
RSC Adv., 2016,
6, 99120-99128
Preferentially grown nanostructured iron disulfide (FeS2) for removal of industrial pollutants†
Received
25th July 2016
, Accepted 7th October 2016
First published on
7th October 2016
Abstract
Preferentially grown nanostructured materials show extraordinary properties even compared with their nanostructured counterparts. The present study deals with a preferentially grown iron disulfide (FeS2) pyrite phase. Pyrite (111) shows high catalytic activity towards methylene blue (MB) and a textile dye (Synazol Yellow K-HL). In the present study, pyrite (111) was successfully synthesized using a low cost effective hydrothermal method and then employed as a photocatalyst for degradation of methylene blue as well as the textile dye Synazol Yellow K-HL. The structural, morphological and optical features of the synthesized FeS2 material were confirmed by X-ray diffraction and UV-visible spectrophotometry. The as synthesized powder was also characterized by Raman spectroscopy to confirm the pyrite phase formation. The dye degradation is based on a mechanism involving reactive oxygen species produced from a photo Fenton like process. The maximum degradation efficiency of a methylene blue dye solution and the textile dye Synazol Yellow K-HL was 95.90% and 99.29%, respectively, after 120 minutes with a 1 g L−1 FeS2 catalyst dose. This work proposes a vision to develop transition metal based photocatalysts for degradation of harmful organic contaminants present in waste water and for many other environment friendly applications.
1. Introduction
Dyes are extensively used in various industries such as cosmetic, food, pharmaceutical and leather industries, but are of major prominence in textile industries. Textile dyes and other industrial dyestuffs introduce to the environment a large number of organochlorine based compounds and heavy metals associated with them, which represent an increasing environmental threat.1,2 The estimated manufacture of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 commercial dyes is roughly 7 × 105 to 1 × 106 tons per year but still there is no exact data on the discharge of dyes to the environment.3 To eliminate these non-biodegradable organic compounds from waste water, biological and physical treatment methods are ineffective for decolourization and degradation of dyes. The drawback of using physical techniques (such as reverse osmosis, ultrafiltration and adsorption on activated carbon) is that they result in secondary pollutants.4 This leads to the need for an appropriate treatment method for dye decolourization and degradation. Recent studies demonstrated that photocatalysis can be used to remove dye effluents efficiently under light irradiation.5 Among the various semiconducting transition metal sulfides, especially those used as photocatalysts for typical organic dyes, FeS2 (pyrite) is an earth abundant and nontoxic semiconductor and has gained significant interest due to its outstanding properties such as electrochemical performance, optical and electronic properties, and degradation ability.6–11 As pyrite is a naturally abundant material, with a suitable band gap (Eg range = 0.8–0.95 eV) and a large absorption coefficient (α < 6 × 105 cm−1 for hν > 1.3 eV), it has the capability to be used in the field of photovoltaics and lithium ion batteries.12 Pyrite is used as a Fenton material for oxidative degradation of organic toxins such as carbon tetrachloride and trichloroethylene, without any contaminated products.13,14 Due to its surface chemical properties, suitable band gap (Eg = 0.95 eV), nontoxic nature, low cost and strong oxidizing ability, pyrite is a potential candidate for a photocatalyst or scavenger for organic pollutants. To synthesize FeS2 nanostructures, various methods have been reported such as a thermal sulphidation/sulfarization process,15,16 chemical vapour deposition,17,18 a hot injection method,19 a hydrothermal method,20,21 a plasma spray process22 and a solvothermal method.23 Among these methods, the hydrothermal method was preferred due to the low temperature of the process which helps to reduce impurities and surface defects. This method has been widely used in synthesis of several metal sulfides with special properties. Dutta et al. investigated the photocatalytic activity of FeS2 for degradation of methylene blue dye (6 × 10−6 M) as well as for rose bengal dye (6.6 × 10−5 M). A sharp decrease in the absorption maxima for both dyes with the passage of irradiation time was observed.24 Liu et al. demonstrated the adsorption and photocatalytic degradation ability of FeS2, synthesized by a solvothermal route, for some typical dyes after variation of different process parameters.25 Bhar et al. reported the degradation of rose bengal dye with mesoporous FeS2. The mesoporous nature of FeS2, having a high surface area, provides adsorption sites for the dyes and enhances the degradation rate.26 Morales et al. demonstrated the photocatalytic activity of FeS2 nanocrystals against methylene blue under UV light. The degradation rate achieved was about 95% in an illumination time of 60 min.27 The aim of this study is to synthesize FeS2 (pyrite) nanostructures and to analyze the degradation kinetics of some typical organic dyes, methylene blue (MB) and a textile dye Synazol Yellow K-HL (SY is used for high light fastness on pale shades).
000 commercial dyes is roughly 7 × 105 to 1 × 106 tons per year but still there is no exact data on the discharge of dyes to the environment.3 To eliminate these non-biodegradable organic compounds from waste water, biological and physical treatment methods are ineffective for decolourization and degradation of dyes. The drawback of using physical techniques (such as reverse osmosis, ultrafiltration and adsorption on activated carbon) is that they result in secondary pollutants.4 This leads to the need for an appropriate treatment method for dye decolourization and degradation. Recent studies demonstrated that photocatalysis can be used to remove dye effluents efficiently under light irradiation.5 Among the various semiconducting transition metal sulfides, especially those used as photocatalysts for typical organic dyes, FeS2 (pyrite) is an earth abundant and nontoxic semiconductor and has gained significant interest due to its outstanding properties such as electrochemical performance, optical and electronic properties, and degradation ability.6–11 As pyrite is a naturally abundant material, with a suitable band gap (Eg range = 0.8–0.95 eV) and a large absorption coefficient (α < 6 × 105 cm−1 for hν > 1.3 eV), it has the capability to be used in the field of photovoltaics and lithium ion batteries.12 Pyrite is used as a Fenton material for oxidative degradation of organic toxins such as carbon tetrachloride and trichloroethylene, without any contaminated products.13,14 Due to its surface chemical properties, suitable band gap (Eg = 0.95 eV), nontoxic nature, low cost and strong oxidizing ability, pyrite is a potential candidate for a photocatalyst or scavenger for organic pollutants. To synthesize FeS2 nanostructures, various methods have been reported such as a thermal sulphidation/sulfarization process,15,16 chemical vapour deposition,17,18 a hot injection method,19 a hydrothermal method,20,21 a plasma spray process22 and a solvothermal method.23 Among these methods, the hydrothermal method was preferred due to the low temperature of the process which helps to reduce impurities and surface defects. This method has been widely used in synthesis of several metal sulfides with special properties. Dutta et al. investigated the photocatalytic activity of FeS2 for degradation of methylene blue dye (6 × 10−6 M) as well as for rose bengal dye (6.6 × 10−5 M). A sharp decrease in the absorption maxima for both dyes with the passage of irradiation time was observed.24 Liu et al. demonstrated the adsorption and photocatalytic degradation ability of FeS2, synthesized by a solvothermal route, for some typical dyes after variation of different process parameters.25 Bhar et al. reported the degradation of rose bengal dye with mesoporous FeS2. The mesoporous nature of FeS2, having a high surface area, provides adsorption sites for the dyes and enhances the degradation rate.26 Morales et al. demonstrated the photocatalytic activity of FeS2 nanocrystals against methylene blue under UV light. The degradation rate achieved was about 95% in an illumination time of 60 min.27 The aim of this study is to synthesize FeS2 (pyrite) nanostructures and to analyze the degradation kinetics of some typical organic dyes, methylene blue (MB) and a textile dye Synazol Yellow K-HL (SY is used for high light fastness on pale shades).
In the present study, pyrite FeS2 nanostructures have been synthesized using iron(II) chloride tetrahydrate (FeCl2·4H2O) as the Fe source and sodium thiosulfate pentahydrate (Na2S2O3·5H2O) as the sulfur source. More importantly, the as prepared FeS2 nanostructures can be used for the photocatalytic degradation of organic and textile dyes. Additionally, the visible light driven photocatalytic performance of FeS2 for organic as well as textile dyes was also evaluated.
2. Experimental
2.1. Materials
The reagents used in this study, iron(II) chloride tetrahydrate (FeCl2·4H2O) and sodium thiosulfate heptahydrate (Na2S2O3·5H2O), were of analytical grade and were purchased from Merck Company. All chemicals were used as received and used without further purification. Distilled water was used in the preparation of all solutions.
2.2. Synthesis of the FeS2 catalyst
Pyrite (FeS2) nanostructures were synthesized by a hydrothermal method. In a typical procedure, 2 g of iron precursor (FeCl2·4H2O, 0.01 M) was dissolved in 40 ml of distilled water, then a 40 ml solution of 8 g of sulfur precursor (Na2S2O3·5H2O, 0.01 M) (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 1
S = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) was added to the iron solution under magnetic stirring. Then, the obtained clear solution was transferred to a Teflon lined stainless steel autoclave, which was sealed and maintained at 200 °C for 24 h. The reaction mixture was then allowed to cool. The precipitates were filtered and washed several times with distilled water, ethanol and carbon disulfide (CS2). The powder was dried under vacuum at 60 °C for 6 h and collected for subsequent characterization.
4) was added to the iron solution under magnetic stirring. Then, the obtained clear solution was transferred to a Teflon lined stainless steel autoclave, which was sealed and maintained at 200 °C for 24 h. The reaction mixture was then allowed to cool. The precipitates were filtered and washed several times with distilled water, ethanol and carbon disulfide (CS2). The powder was dried under vacuum at 60 °C for 6 h and collected for subsequent characterization.
2.3. Characterization
X-ray diffraction (XRD) patterns of the samples were obtained from a PANalytical X-ray diffractometer (XRD) using Cu Kα radiation (λ = 1.54056 Å) with a 2θ range from 25° to 70°. Raman measurements were conducted using a Renishaw spectrometer with a laser (514 nm) for excitation. UV-visible spectroscopy measurements were conducted using a Shimadzu UV-2600 over a wavelength range of 300–800 nm.
2.4. Photocatalysis experiment
The photocatalytic activities of the samples were determined via the photocatalytic degradation of methylene blue (MB) and the textile dye Synazol Yellow K-HL (SY) under visible light irradiation. A 100 W halogen lamp was used as a visible light energy source for catalyst excitation. The photon flux of visible radiation was measured to be 4.17 × 10−4 mol of photons per second. Methylene blue (a cationic dye) is a water soluble dye that was used as an ideal compound for the oxidation reactions of environmental organic pollutants. Synazol Yellow (an azo dye) is a water soluble dye that is mostly used for cotton dyeing and contains both the sulphatoethylsulphone group (SES) and the monochlorotriazine (MCT) group. Therefore, Synazol Yellow was chosen as the representative azo textile dye for catalysis investigation. The chemical structures of the used dyes are given in Fig. 1. The experiments were carried out at room temperature, and a known amount (50 mg) of photocatalyst FeS2 (1 g L−1) powder was dissolved into 50 ml of two different dye solutions (1 mg L−1) of methylene blue and Synazol Yellow K-HL. Before illumination, the suspension was sonicated for 30 min then magnetically stirred for 40 minutes in darkness, to achieve adsorption equilibrium to reduce the error due to any initial adsorption effect. Then the mixed system was irradiated using the visible light source for a given time duration. At certain time intervals, 5 ml of suspension was collected, centrifuged and analyzed using UV-visible spectroscopy (Shimadzu, UV-2600). To determine the dye concentration, based on the Beer–Lambert law, the curves were studied to correlate the absorbance with respect to the maximum absorption wavelengths (i.e. 664 nm for MB and 416 nm for SY).
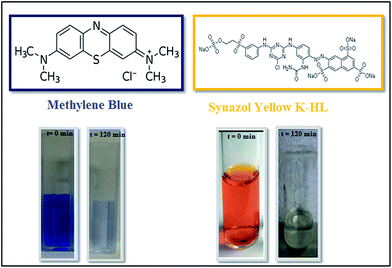 |
| | Fig. 1 Structures of the organic dye methylene blue as well as the textile dye Synazol Yellow, and their decolourization with time. | |
3. Result and discussion
3.1. Characterization of the FeS2 nanostructures
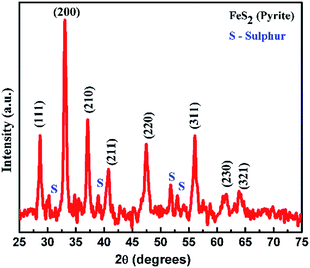
The X-ray diffraction (XRD) pattern of the as prepared FeS2 is shown in Fig. 2. All peaks of the as prepared FeS2 can be assigned to the standard values of cubic FeS2 pyrite with lattice constants a = b = c = 5.414 Å (JCPDS no. 024-0076 a = 5.418 Å).28 Some of the peaks in the XRD pattern revealed the presence of unreacted sulfur. No peaks are observed for impurities such as FeS, FeSO4, or FeSx in the as obtained FeS2 sample, indicating the high purity of the products. The crystal structure of FeS2 (pyrite) is shown in Fig. 3. The positions of the atoms as well as the crystal structure parameters are listed in Table S2.† The particle size of the as obtained sample was calculated, using the XRD pattern, by the Hall Williamson formula i.e.| | β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/λ = 1/L + ε θ/λ = 1/L + ε![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/λ θ/λ | (1) |
where β is the full width half maximum of the diffraction peaks, ε is the strain and L is the crystallite size.
 |
| | Fig. 2 XRD pattern of the as synthesized pyrite FeS2 nanoparticles. | |
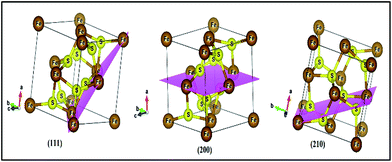 |
| | Fig. 3 Crystal structure of FeS2 (pyrite) with (111), (200), and (210) planes. Iron and sulfur atoms are coloured brown and yellow, respectively. | |
Fig. S1 (in ESI†) shows the plot of (sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) versus (β
θ) versus (β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ), which is a straight line. The average crystallite size of the as prepared sample can be calculated from the intercept of the β
θ), which is a straight line. The average crystallite size of the as prepared sample can be calculated from the intercept of the β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ axis, which is equal to λ/L, i.e. ∼14 nm.29 The strain present in the as prepared sample can be evaluated using the slope of the plot of Fig. S1† and is determined to be 0.0027. The positive value of the slope indicates that the tensile strain occurs in the crystal lattice, which is also confirmed by the small shift of the diffraction peaks towards lower diffraction angles. The texture coefficient was calculated by using the following equation:30
θ axis, which is equal to λ/L, i.e. ∼14 nm.29 The strain present in the as prepared sample can be evaluated using the slope of the plot of Fig. S1† and is determined to be 0.0027. The positive value of the slope indicates that the tensile strain occurs in the crystal lattice, which is also confirmed by the small shift of the diffraction peaks towards lower diffraction angles. The texture coefficient was calculated by using the following equation:30
| | 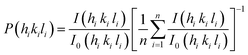 | (2) |
where
P(
hikili) is the texture coefficient of the plane specified by the Miller indices (
hikili);
I(
hikili) and
I0(
hikili) are the specimen and standard intensities respectively for a given peak and ‘
n’ is the number of diffraction peaks.
The texture coefficient for particular planes and the corresponding crystallite size is tabulated in Table S1.† For a randomly distributed powder sample, the value of the texture coefficient is close to unity, while for a preferred orientation in a plane, the value of the texture coefficient is greater than 1. The texture analysis reveals that the sample is highly textured along the (111) plane as shown in Fig. 3. For the (111) plane, there is a contribution from the Fe atoms only for surrounding neighbours. But for other planes such as (200) and (210), sulfur as well as iron atoms contribute and this results in a closely packed dense structure. So, (111) is the preferentially grown plane with the lowest packing density and the most available active sites, compared to the other planes, as shown in Fig. 3. As the grain number of the preferred orientation in a specific plane is higher compared to other planes, this leads to a high surface area of FeS2 with a large number of active sites, resulting in increased production of OH˙ radicals and therefore increased degradation of dyes during photocatalysis.31,32
3.2. Raman spectroscopy
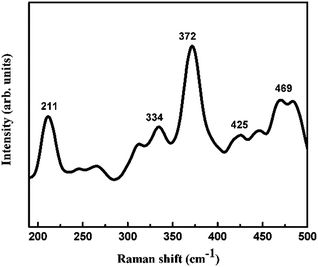
Fig. 4 shows a Raman spectrum of the FeS2 nanostructures. There is a strong peak observed at 372 cm−1 which is the characteristic active Ag mode of pyrite FeS2. This peak is attributed to the S–S in phase stretching vibration in which sulphur atoms substitute vertically along the molecular axis. The Eg stretch mode peak at 334 cm−1 is due to displacement of sulphur atoms within the S–S bond.33,34 The weak peak at 425 cm−1 indicates coupled liberation and the Tg stretch mode. Additional peaks are observed at 211 cm−1and 469 cm−1, which are clearly assigned to elemental sulphur.35
 |
| | Fig. 4 Raman spectroscopy of the as synthesized pyrite FeS2 nanostructures. | |
3.3. Mechanism for dye degradation by pyrite based photocatalysts
In this article, the photocatalytic performance of FeS2 was evaluated using some typical dyes such as methylene blue and textile dyes such as Synazol Yellow. The schematic representation of the mechanism for dye degradation is shown in Fig. 5. The FeS2 material can absorb visible light using the mechanism described in Fig. 5. The release of iron species such as Fe2+ and Fe3+ can occur due to facile oxidation of FeS2 (pyrite) under ambient air conditions.36 Oxygen helps in the generation of reactive oxygen species such as superoxide radicals, hydrogen peroxide and hydroxyl radicals via accepting an electron from ferrous ions.37 The more reactive oxygen species can be produced by inducing FeS2 pyrite catalysis using visible light irradiation. The hydroxyl radicals result from the transformation of products i.e. hydrogen peroxide (H2O2) and the superoxide radical. The reactive oxygen species OH˙ induces the attack on the dye molecules (methylene blue and Synazol Yellow).38,39 Consequently, decomposition of the dye molecules (i.e. methylene blue MB and Synazol Yellow SY) results in a series of intermediates with smaller molecular sizes. Lastly, the mineralization of these intermediates could be to carbon dioxide (CO2) and water (H2O). Thus, degradation of the dye was achieved via a Fenton like process and a sequence of degradation procedures can be described in the following reactions (eqn (3)–(11)).| | | FeS2 + hν → ecb− + hvb+ | (3) |
| | | 2FeS2 + 7O2 + 2H2O → 2Fe2+ + 4H+ + 4SO42− | (4) |
| | | Fe2+ + O2 → Fe3+ + O2˙− | (5) |
| | | Fe2+ + O2˙− + 2H+ → Fe3+ + H2O2 | (7) |
| | | 2O2˙− + 2H+ → O2 + H2O2 | (8) |
| | | H2O2 + ecb− → OH˙ + OH− | (9) |
| | | Fe2+ + H2O2 → Fe3+ + OH˙ + OH− | (11) |
| | | Fe3+ + H2O → Fe2+ + OH˙ + H+ | (12) |
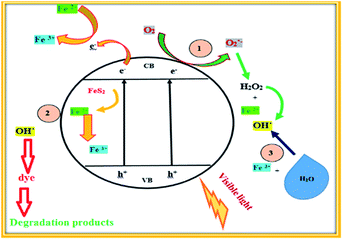 |
| | Fig. 5 Schematic representation of the dye degradation mechanism of the FeS2 material. | |
Fe2+ ions were formed by inducing FeS2 pyrite catalysis with the help of water (H2O) and H+.40 Further, the Fe2+ species transformed into Fe3+ ions on the surface of pyrite. Finally, the as formed Fe3+ species could react with water (H2O) to form OH˙ (eqn (12)). Thus, the dye was degraded by forming the OH˙ radical (eqn (13)).
| | | Dye + OH˙ → degradation | (13) |
The Fenton process is an advanced oxidation process which utilizes ferrous ions and hydrogen peroxide to produce the second most powerful oxidant i.e. the hydroxyl radical (OH˙) in aqueous solution.41 In order to estimate the photocatalytic activities of the as synthesized FeS2 nanostructures, the degradation of a widely used dye, methylene blue (MB), in aqueous solution under visible light irradiation was studied. The change in concentration of methylene blue (MB) and Synazol Yellow (SY) dye was given by the degradation ratio of both dyes and was calculated using the following formula:
| | 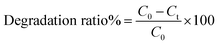 | (14) |
where
C0 and
Ct are the concentrations of methylene blue (MB) or Synazol Yellow (SY) dye before and after the reaction.
| | | OH˙ + Fe2+ → OH− + Fe3+ | (15) |
Fig. 6a and b, 7a and b and 8 show a sequence of UV-vis absorption spectra for the methylene blue (MB) dye (1 mg L−1) suspension in the presence of the different concentration loading of the FeS2 catalyst exposed under visible light for up to 120 min at constant time intervals. MB exhibits a strong absorption band centered at 664 nm and a shoulder at 610 nm. Both absorption bands gradually decreased as the visible light irradiation time increased, which showed the decomposition of the MB chromophoric structure. The catalytic performance of pyrite in the degradation of the methylene blue dye solution was studied to investigate the effect of concentration on the FeS2 catalytic performance, as discussed later. Fig. 9a represents the degradation efficiency of methylene blue dye as a function of time for different loadings of pyrite in the aqueous solution. The methylene blue degradation efficiency improved from 54.06% to 95.9% after 120 min when the initial dosage of pyrite was varied from 0.02 g L−1 to 1 g L−1. It is found that the 1 g L−1 FeS2 catalyst loading shows much more impressive photocatalytic activity under visible light than other concentrations of FeS2 catalyst. As the concentration of FeS2 pyrite was increased, the number of active sites on the surface of pyrite was also increased for reactive radical formation, which revealed the enhanced degradation efficiency with high concentrations of FeS2 catalyst.42,43 Afterwards, there was an opposite trend observed which thus indicated a decrease in the degradation efficiency. The decrement in the degradation efficiency may be due to concentrated iron species scavenging reactive radicals (eqn (15)), which results in reduction of the amount of hydroxyl radicals which leads to the decreased degradation efficiency.44 The photodegradation kinetics of the dye were evaluated by observing the absorption spectra of the MB dye solution at an absorption maximum wavelength of 664 nm. The photodegradation efficiency was calculated for different catalyst concentrations (Fig. 9b) for methylene blue dye (1 mg L−1) in a histogram representation. The results indicate that the rate of degradation for each concentration of catalyst increased with time. The higher degradation rate results in the faster degradation of dye. The average decolorization rate was calculated using the formula as follows:
| | 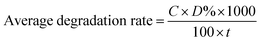 | (16) |
where
C = initial concentration of dye solution, and
D% = dye degradation after time
t.
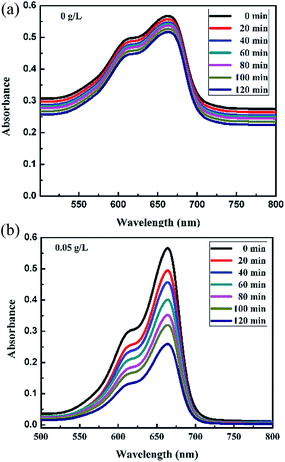 |
| | Fig. 6 Absorption spectra changes for the MB (1 mg L−1) aqueous solution in the presence of (a) 0 g L−1 and (b) 0.05 g L−1 loading of FeS2 under visible light. | |
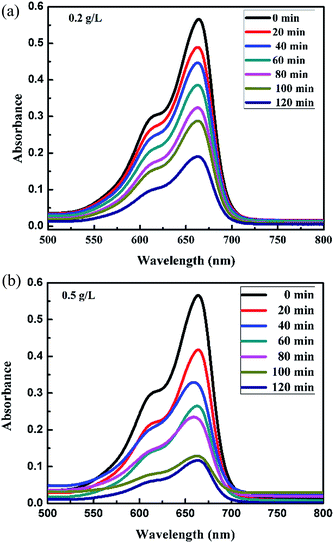 |
| | Fig. 7 Absorption spectra changes for the MB (1 mg L−1) aqueous solution in the presence of (a) 0.2 g L−1 and (b) 0.5 g L−1 loading of FeS2 under visible light. | |
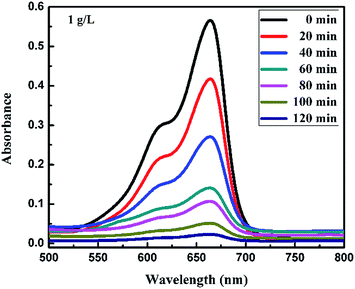 |
| | Fig. 8 Absorption spectra changes for the MB (1 mg L−1) aqueous solution in the presence of 1 g L−1 loading of FeS2 under visible light. | |
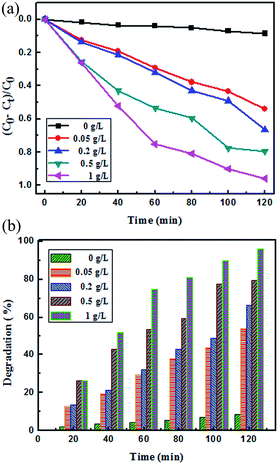 |
| | Fig. 9 (a) Photocatalytic degradation efficiency of different concentrations of FeS2 catalyst loading with methylene blue (1 mg L−1) dye solution; (b) comparison of the photocatalytic activity of different concentration loadings of catalyst FeS2 under visible light irradiation. | |
For a 1 g L−1 FeS2 (pyrite) catalyst dose for methylene blue dye (1 mg L−1), the average rate of degradation was 7.991%, approximately. The half life of the dye, which is the time required for the MB concentration to decrease by half, was determined from the intersection point of the curves for changes in MB concentration (C/C0) and degradation efficiency (1 − C/C0). As the FeS2/MB concentration ratio increased, the photodegradation efficiency increased and half life decreased on the basis of the curves (Fig. 10 and Table 1).
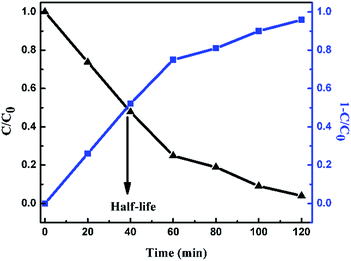 |
| | Fig. 10
C/C0 curve and degradation efficiency (1 − C/C0) curve for 1 g L−1 of FeS2 catalyst loading into 1 mg L−1 MB dye solution. | |
Table 1 Photodegradation data of MB and SY assisted by FeS2 under visible light irradiation
| Dye |
FeS2/dye |
Half-life (min) |
Degradation efficiency (%) |
| Methylene blue (MB) |
0 g L−1 |
— |
8.48 |
| 0.05 g L−1 |
60 |
54.06 |
| 0.2 g L−1 |
60 |
66.40 |
| 0.5 g L−1 |
60 |
79.32 |
| 1 g L−1 |
40 |
95.90 |
| Synazol Yellow K-HL (SY) |
0 g L−1 |
— |
27.27 |
| 0.05 g L−1 |
81 |
94.55 |
| 0.2 g L−1 |
81 |
96.13 |
| 0.5 g L−1 |
81 |
97.18 |
| 1 g L−1 |
54.14 |
99.29 |
For the Synazol Yellow K-HL dye, the absorption peak was observed at 416 nm due to the azo structure of the dye,2 and is shown as a function of time for different concentrations of FeS2 (pyrite) in Fig. 11a and b, 12a and b and 13. The intensity of the absorption peaks diminished as the irradiation time of visible light increased from 0 to 120 minutes.
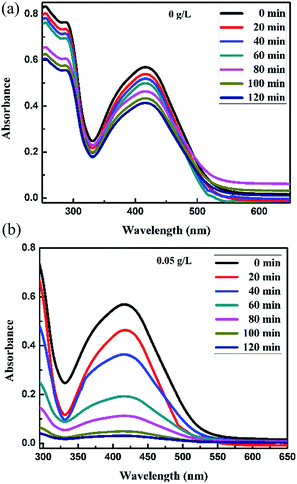 |
| | Fig. 11 Absorption spectra changes for the textile dye Synazol Yellow K-HL (1 mg L−1) aqueous solution in the presence of (a) 0 g L−1 and (b) 0.05 g L−1 concentrations of FeS2 under visible light. | |
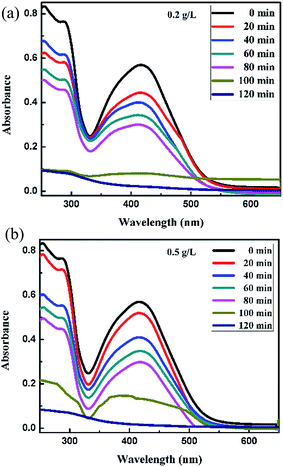 |
| | Fig. 12 Absorption spectra changes for the textile dye Synazol Yellow K-HL (1 mg L−1) aqueous solution in the presence of (a) 0.2 g L−1 and (b) 0.5 g L−1 concentrations of FeS2 under visible light. | |
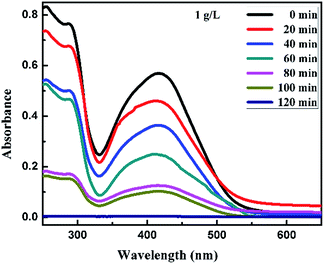 |
| | Fig. 13 Absorption spectra changes for the textile dye Synazol Yellow K-HL (1 mg L−1) aqueous solution in the presence of 1.0 g L−1 concentration of FeS2 under visible light. | |
These results revealed that the azo bonds of the Synazol Yellow K-HL dye decomposed under visible light irradiation in the presence of FeS2 pyrite. The degradation efficiency reached 99.29% after 120 min (Fig. 14a and Table 1) with 1 g L−1 catalyst FeS2 loading. The degradation efficiency of different concentrations of FeS2 catalyst loadings for Synazol Yellow dye (1 mg L−1) is shown in Fig. 14b. The average rate of degradation was calculated to be approximately 8.274% for a 1 g L−1 FeS2 catalyst concentration with Synazol Yellow dye (using eqn (14)), which is further confirmed by the histogram pictorial representation (Fig. 14b). The maximum degradation efficiency leads to high degradation of textile dyes used in many industries, which helps in waste water treatment and hence reduces water contamination.
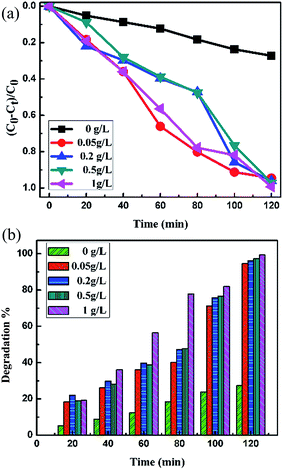 |
| | Fig. 14 (a) Photocatalytic degradation efficiency of different concentrations of FeS2 catalyst loading of Synazol Yellow K-HL (1 mg L−1) dye solution; (b) comparison of the photocatalytic activity of different concentration loadings of catalyst FeS2 under visible light irradiation for Synazol Yellow K-HL dye. | |
The half-life calculation for Synazol dye was determined to be 54.14 min for the 1 g L−1 FeS2 catalyst dose (Fig. 15 and Table 1). Also in this case, beyond a certain limit of catalyst concentration, the degradation percentage starts to decrease as the solution becomes turbid, as this results in blockage of visible light, due to which the reaction is suppressed.45
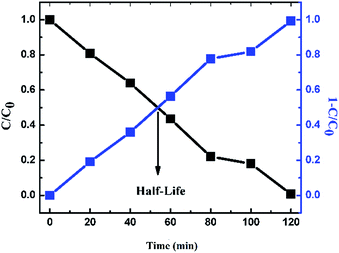 |
| | Fig. 15
C/C0 curve and degradation efficiency (1 − C/C0) for 1 g L−1 of FeS2 catalyst loading into 1 mg L−1 Synazol Yellow K-HL dye solution. | |
4. Conclusion
FeS2 (pyrite) is a potential photocatalyst for degradation of methylene blue (MB) and the textile dye Synazol Yellow K-HL (SY). The results indicate that the preferentially grown pyrite (111) (FeS2) acts as a photocatalyst and its degradation ratio is nearly 95.90% and 98.94% within 120 minutes towards methylene blue and Synazol Yellow K-HL textile dye, respectively, under visible light irradiation. The as prepared FeS2 has good catalytic and optical properties. Thus, FeS2 was used as a photocatalyst with differing dose/load of the catalyst. In conclusion, pyrite has been successfully used as a Fenton catalyst for the oxidative degradation of various contaminants. The excellent photocatalytic performance of FeS2/MB indicates that FeS2 acts as a promising support, which should be used as an important guide to further design more useful photocatalytic systems for the removal of toxic dyes or pollutants.
Acknowledgements
This work was financially supported through Maulana Azad National Fellowship under Ministry of Minority Affairs, India (MoMA) for Minority Students (MANF-2014-15-SIK-PUN-36753). Also, the authors acknowledge the financial support of BRNS project (Board of Research in Nuclear Sciences, DAE, India) (project no. 34/14/41/2014-BRNS/2035).
Notes and references
- H. Lachheb, E. Puzenat, A. Houas, M. Ksibi, E. Elaloui, C. Guillard and J. M. Herrmann, Appl. Catal., B, 2002, 39, 75–90 CrossRef CAS.
- A. A. P. Mansur, H. S. Mansur, F. P. Ramanery, L. C. Oliveira and P. P. Souza, Appl. Catal., B, 2014, 158, 269–279 CrossRef.
-
K. Hunger, Industrial Dyes: Chemistry, Properties, Applications, Wiley – VCH, Weinheim, Cambridge, 2003, ISBN no. 9783527304264 Search PubMed.
- A. Ahmad, S. H. M. Setapar, C. S. Chuang, A. Khatoon, W. A. Wani, R. Kumar and M. Rafatullah, RSC Adv., 2015, 5, 30801–30818 RSC.
- G. Moussavi and M. Mahmoudi, Chem. Eng. J., 2009, 152, 1–7 CrossRef CAS.
- A. Ennaoui, S. Fiechter, H. Goslowsky and H. Tributsch, J. Electrochem. Soc., 1985, 132, 1579–1582 CrossRef CAS.
- Y. Bi, Y. Yuan, C. L. Exstrom, S. A. Darveau and I. Huang, Nano Lett., 2012, 11, 4953–4957 CrossRef PubMed.
- Y. Wang, X. Qian, W. Zhou, H. Liao and S. Cheng, RSC Adv., 2014, 4, 36597–36602 RSC.
- H. A. Macpherson and C. R. Stoldt, ACS Nano, 2012, 6, 8940–8949 CrossRef CAS PubMed.
- M. Cabàn-Acevedo, M. S. Faber, Y. Tan, R. J. Hamers and S. Jin, Nano Lett., 2012, 12, 1977–1982 CrossRef PubMed.
- S. Liu, M. Li, S. Li, H. Li and L. Yan, Appl. Surf. Sci., 2013, 268, 213–217 CrossRef CAS.
- M. C. Acevedo, N. S. Kaiser, C. R. English, D. Liang, B. J. Thompson, H. E. Chen, K. J. Czech, J. C. Wright, R. J. Hamers and S. Jin, J. Am. Chem. Soc., 2014, 136, 17163 CrossRef PubMed.
- H. Che, S. Bae and W. Lee, J. Hazard. Mater., 2011, 185, 1355–1361 CrossRef CAS PubMed.
- H. Che and W. Lee, Chemosphere, 2011, 82, 103–108 CrossRef PubMed.
- V. G. Bessergenev, R. J. F. Pereira and A. M. Botelho de Rego, Surf. Coat. Technol., 2007, 201, 9141–9145 CrossRef CAS.
- R. Morrish, R. Silverstein and C. A. Wolden, J. Am. Chem. Soc., 2012, 134, 17854–17857 CrossRef CAS PubMed.
- H. Xian, J. Zhu, X. Liang and H. He, RSC Adv., 2016, 6, 31998–31999 Search PubMed.
- L. Samad, M. C. Acevedo, M. J. Shearer, K. Park, R. J. Hamers and S. Jin, Chem. Mater., 2015, 27, 3108–3114 CrossRef.
- J. Puthussery, S. Seefeld, N. Berry, M. Gibbs and M. Law, J. Am. Chem. Soc., 2011, 133, 716–719 CrossRef CAS PubMed.
- C. Wadia, Y. Wu, S. Gul, S. K. Volkman, J. Guo and A. P. Alivisatos, Chem. Mater., 2009, 21, 2568–2570 CrossRef CAS.
- Y. Liang, P. Bai, J. Zhou, T. Wang, B. Luo and S. Zhang, CrystEngComm, 2016, 18, 6262–6271 RSC.
- H. D. Wang, B. S. Xu, J. J. Liu, D. M. Zhuang, S. C. Wei and G. Jin, Surf. Coat. Technol., 2007, 201, 5286–5289 CrossRef CAS.
- S. Kar and S. Chaudhuri, Mater. Lett., 2005, 59, 289–292 CrossRef CAS.
- A. K. Dutta, S. K. Maji, D. N. Srivastava, A. Mondal, P. Biswas, P. Paul and B. Adhikary, ACS Appl. Mater. Interfaces, 2012, 4, 1919–1927 CAS.
- S. Liu, M. Li, S. Li, H. Li and L. Yan, Appl. Surf. Sci., 2013, 268, 213–217 CrossRef CAS.
- S. K. Bhar, S. Jana, A. Mondal and N. Mukherjee, J. Colloid Interface Sci., 2013, 393, 286–290 CrossRef CAS PubMed.
- M. V. Morales-Gallardo, A. M. Ayala, M. Pal, M. A. Cortes, J. A. Toledo and N. R. Matehews, Chem. Phys. Lett., 2016, 660, 93–98 CrossRef CAS.
- S. Liu, J. Wu, P. Yu, Q. Ding, Z. Zhou, H. Li, C. Lai, Y. L. Chueh and Z. M. Wang, Nanoscale Res. Lett., 2014, 9, 549–556 CrossRef PubMed.
- J. S. Parramon, V. Janicki and H. Zorc, Thin Solid Films, 2008, 516, 5478–5482 CrossRef.
- A. Kumar, K. Singh and O. P. Pandey, J. Mater. Sci. Technol., 2014, 30, 112–116 CAS.
- F. Zaera, Surf. Sci., 2002, 500, 947–965 CrossRef CAS.
- C. Gregor, M. Hermanek, D. Janick, J. Pechousek, J. Filip, J. Hrbac and R. Zboril, Eur. J. Inorg. Chem., 2010, 2010, 2343–2351 CrossRef.
- H. Xue, D. Y. W. Yu, J. Qing, X. Yang, J. Xu, Z. Li, M. Sun, W. Kang, Y. Tang and C. S. Lee, J. Mater. Chem. A, 2015, 3, 7945–7949 CAS.
- W. L. Liu, X. H. Rui, H. T. Tan, C. Xu, Q. Y. Yan and H. H. Hng, RSC Adv., 2014, 4, 48770–48776 RSC.
- V. Toniazzo, C. Mustin, J. M. Portal, B. Humbert, R. Benoit and R. Erre, Appl. Surf. Sci., 1999, 143, 229–237 CrossRef CAS.
- J. E. Biaglow and A. V. Kachur, J. Radiat. Res., 1997, 148, 181–187 CrossRef CAS.
- Y. Q. Zhang, H. P. Tran, I. Hussain, Y. Q. Zhou and S. B. Huang, Chem. Eng. J., 2015, 279, 396–401 CrossRef CAS.
- Z. H. Diao, M. Y. Li, F. Y. Zeng, L. Sang and R. L. Qiu, J. Hazard. Mater., 2013, 260, 585–592 CrossRef CAS PubMed.
- Z. H. Diao, X. R. Xu, F. M. Liu, Y. X. Sun, Z. W. Zhang, K. F. Sun, S. Z. Wang and H. Cheng, Sep. Purif. Technol., 2015, 154, 168–175 CrossRef CAS.
- A. Tian, Q. Xu, X. Shi, H. Yang, X. Xue, J. You, X. Wang, C. Dong, X. Yan and H. Zhou, RSC Adv., 2015, 5, 62724–62731 RSC.
- I. A. Balachioglu, I. Arslan and M. T. Sacan, Environ. Technol., 2001, 22, 813–822 CrossRef PubMed.
- X. Zhang, Y. Ding, H. Tang, X. Han, L. Zhu and N. Wang, Chem. Eng. J., 2014, 236, 251–262 CrossRef CAS.
- A. Khataee, P. Gholami, M. Sheydaei, S. Khorram and S. W. Joo, New J. Chem., 2016, 40, 5221–5230 RSC.
- Y. Kuang, Q. Wang, Z. Chen, M. Megharaj and R. Naidu, J. Colloid Interface Sci., 2013, 410, 67–73 CrossRef CAS PubMed.
- M. A. Rauf and S. S. Ashraf, Chem. Eng. J., 2009, 151, 10–18 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra18838a |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 commercial dyes is roughly 7 × 105 to 1 × 106 tons per year but still there is no exact data on the discharge of dyes to the environment.3 To eliminate these non-biodegradable organic compounds from waste water, biological and physical treatment methods are ineffective for decolourization and degradation of dyes. The drawback of using physical techniques (such as reverse osmosis, ultrafiltration and adsorption on activated carbon) is that they result in secondary pollutants.4 This leads to the need for an appropriate treatment method for dye decolourization and degradation. Recent studies demonstrated that photocatalysis can be used to remove dye effluents efficiently under light irradiation.5 Among the various semiconducting transition metal sulfides, especially those used as photocatalysts for typical organic dyes, FeS2 (pyrite) is an earth abundant and nontoxic semiconductor and has gained significant interest due to its outstanding properties such as electrochemical performance, optical and electronic properties, and degradation ability.6–11 As pyrite is a naturally abundant material, with a suitable band gap (Eg range = 0.8–0.95 eV) and a large absorption coefficient (α < 6 × 105 cm−1 for hν > 1.3 eV), it has the capability to be used in the field of photovoltaics and lithium ion batteries.12 Pyrite is used as a Fenton material for oxidative degradation of organic toxins such as carbon tetrachloride and trichloroethylene, without any contaminated products.13,14 Due to its surface chemical properties, suitable band gap (Eg = 0.95 eV), nontoxic nature, low cost and strong oxidizing ability, pyrite is a potential candidate for a photocatalyst or scavenger for organic pollutants. To synthesize FeS2 nanostructures, various methods have been reported such as a thermal sulphidation/sulfarization process,15,16 chemical vapour deposition,17,18 a hot injection method,19 a hydrothermal method,20,21 a plasma spray process22 and a solvothermal method.23 Among these methods, the hydrothermal method was preferred due to the low temperature of the process which helps to reduce impurities and surface defects. This method has been widely used in synthesis of several metal sulfides with special properties. Dutta et al. investigated the photocatalytic activity of FeS2 for degradation of methylene blue dye (6 × 10−6 M) as well as for rose bengal dye (6.6 × 10−5 M). A sharp decrease in the absorption maxima for both dyes with the passage of irradiation time was observed.24 Liu et al. demonstrated the adsorption and photocatalytic degradation ability of FeS2, synthesized by a solvothermal route, for some typical dyes after variation of different process parameters.25 Bhar et al. reported the degradation of rose bengal dye with mesoporous FeS2. The mesoporous nature of FeS2, having a high surface area, provides adsorption sites for the dyes and enhances the degradation rate.26 Morales et al. demonstrated the photocatalytic activity of FeS2 nanocrystals against methylene blue under UV light. The degradation rate achieved was about 95% in an illumination time of 60 min.27 The aim of this study is to synthesize FeS2 (pyrite) nanostructures and to analyze the degradation kinetics of some typical organic dyes, methylene blue (MB) and a textile dye Synazol Yellow K-HL (SY is used for high light fastness on pale shades).
000 commercial dyes is roughly 7 × 105 to 1 × 106 tons per year but still there is no exact data on the discharge of dyes to the environment.3 To eliminate these non-biodegradable organic compounds from waste water, biological and physical treatment methods are ineffective for decolourization and degradation of dyes. The drawback of using physical techniques (such as reverse osmosis, ultrafiltration and adsorption on activated carbon) is that they result in secondary pollutants.4 This leads to the need for an appropriate treatment method for dye decolourization and degradation. Recent studies demonstrated that photocatalysis can be used to remove dye effluents efficiently under light irradiation.5 Among the various semiconducting transition metal sulfides, especially those used as photocatalysts for typical organic dyes, FeS2 (pyrite) is an earth abundant and nontoxic semiconductor and has gained significant interest due to its outstanding properties such as electrochemical performance, optical and electronic properties, and degradation ability.6–11 As pyrite is a naturally abundant material, with a suitable band gap (Eg range = 0.8–0.95 eV) and a large absorption coefficient (α < 6 × 105 cm−1 for hν > 1.3 eV), it has the capability to be used in the field of photovoltaics and lithium ion batteries.12 Pyrite is used as a Fenton material for oxidative degradation of organic toxins such as carbon tetrachloride and trichloroethylene, without any contaminated products.13,14 Due to its surface chemical properties, suitable band gap (Eg = 0.95 eV), nontoxic nature, low cost and strong oxidizing ability, pyrite is a potential candidate for a photocatalyst or scavenger for organic pollutants. To synthesize FeS2 nanostructures, various methods have been reported such as a thermal sulphidation/sulfarization process,15,16 chemical vapour deposition,17,18 a hot injection method,19 a hydrothermal method,20,21 a plasma spray process22 and a solvothermal method.23 Among these methods, the hydrothermal method was preferred due to the low temperature of the process which helps to reduce impurities and surface defects. This method has been widely used in synthesis of several metal sulfides with special properties. Dutta et al. investigated the photocatalytic activity of FeS2 for degradation of methylene blue dye (6 × 10−6 M) as well as for rose bengal dye (6.6 × 10−5 M). A sharp decrease in the absorption maxima for both dyes with the passage of irradiation time was observed.24 Liu et al. demonstrated the adsorption and photocatalytic degradation ability of FeS2, synthesized by a solvothermal route, for some typical dyes after variation of different process parameters.25 Bhar et al. reported the degradation of rose bengal dye with mesoporous FeS2. The mesoporous nature of FeS2, having a high surface area, provides adsorption sites for the dyes and enhances the degradation rate.26 Morales et al. demonstrated the photocatalytic activity of FeS2 nanocrystals against methylene blue under UV light. The degradation rate achieved was about 95% in an illumination time of 60 min.27 The aim of this study is to synthesize FeS2 (pyrite) nanostructures and to analyze the degradation kinetics of some typical organic dyes, methylene blue (MB) and a textile dye Synazol Yellow K-HL (SY is used for high light fastness on pale shades).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 1
S = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) was added to the iron solution under magnetic stirring. Then, the obtained clear solution was transferred to a Teflon lined stainless steel autoclave, which was sealed and maintained at 200 °C for 24 h. The reaction mixture was then allowed to cool. The precipitates were filtered and washed several times with distilled water, ethanol and carbon disulfide (CS2). The powder was dried under vacuum at 60 °C for 6 h and collected for subsequent characterization.
4) was added to the iron solution under magnetic stirring. Then, the obtained clear solution was transferred to a Teflon lined stainless steel autoclave, which was sealed and maintained at 200 °C for 24 h. The reaction mixture was then allowed to cool. The precipitates were filtered and washed several times with distilled water, ethanol and carbon disulfide (CS2). The powder was dried under vacuum at 60 °C for 6 h and collected for subsequent characterization.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/λ = 1/L + ε
θ/λ = 1/L + ε![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/λ
θ/λ
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) versus (β
θ) versus (β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ), which is a straight line. The average crystallite size of the as prepared sample can be calculated from the intercept of the β
θ), which is a straight line. The average crystallite size of the as prepared sample can be calculated from the intercept of the β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ axis, which is equal to λ/L, i.e. ∼14 nm.29 The strain present in the as prepared sample can be evaluated using the slope of the plot of Fig. S1† and is determined to be 0.0027. The positive value of the slope indicates that the tensile strain occurs in the crystal lattice, which is also confirmed by the small shift of the diffraction peaks towards lower diffraction angles. The texture coefficient was calculated by using the following equation:30
θ axis, which is equal to λ/L, i.e. ∼14 nm.29 The strain present in the as prepared sample can be evaluated using the slope of the plot of Fig. S1† and is determined to be 0.0027. The positive value of the slope indicates that the tensile strain occurs in the crystal lattice, which is also confirmed by the small shift of the diffraction peaks towards lower diffraction angles. The texture coefficient was calculated by using the following equation:30