Synergistic nanofibrous adsorbent for uranium extraction from seawater†
Bowu Zhang‡
 *a,
Xiaojing Guo‡a,
Siyuan Xieab,
Xiyan Liua,
Changjian Linga,
Hongjuan Maa,
Ming Yua and
Jingye Li*a
*a,
Xiaojing Guo‡a,
Siyuan Xieab,
Xiyan Liua,
Changjian Linga,
Hongjuan Maa,
Ming Yua and
Jingye Li*a
aCAS Centre for Innovation in Advanced Nuclear Energy, Shanghai Institute of Applied Physics, Chinese Academy of Sciences, No. 2019 Jialuo Rd., Jiading Dist., Shanghai, 201800, China. E-mail: zhangbowu@foxmail.com; jingyeli@sinap.ac.an; Fax: +86-21-39494505; Tel: +86-21-39494505
bPatent Examination Cooperation Centre of the Patent Office, State Intellectual Property Office of the P. R. China, No. 55 Fengchan Rd., Jinshui Dist., Zhengzhou, 450002, China
First published on 22nd August 2016
Abstract
Huge reserves of uranium (U) in seawater have been of interest to scientists and energy companies since the 1950s. However, extracting trace concentrations (3.3 ppb) of U from seawater is economically unfeasible without new, high-performance adsorbents. Here, a mat-like nanofibrous composite adsorbent containing binary coordination groups (amidoxime (AO) and carboxyl (AC−)) in a highly porous network of nanofibers is constructed via a parallel-blend electrospinning method. Its U uptake in artificial seawater is more than double those of adsorbents containing AO or AC− groups alone. Density functional theory (DFT) calculations reveal that this synergistic effect is because the AC− group promotes both the U 5f/6d orbital contribution to U–AO bonding and the dissociation of uranyl tricarbonate ions in seawater. In a continuous flow-through experiment with simulated seawater, the nanofibrous adsorbent achieves an adsorption capacity up to 2.86 mg U gads−1 in 30 d but without saturation, indicating a high efficiency for U extraction.
Introduction
As the total U reserve in seawater far exceeds the terrestrial reserve, U extracted from seawater could be a crucial resource for nuclear energy development in the future.1,2 However, the concentration of U in seawater is minuscule (3.3 ppb U), and it is accompanied by much higher concentrations of other metal ions, including vanadium (V), iron (Fe), copper (Cu), magnesium (Mg), and calcium (Ca). Therefore, it is important to develop highly effective adsorbents for this purpose.2,3 Various adsorbents have been considered since the 1950s. The earliest candidates, namely inorganic mineral adsorbents such as hydrous titanium oxide and galena,4,5 exhibit very poor U uptake and are difficult to recycle in the field. Recently, polyethylene fibre/fabric-based adsorbents containing the amidoxime (AO) group were found to exhibit good mechanical strength and selective adsorption of U in marine experiments, which have been conducted offshore of Sekine-Hama in Aomori Prefecture6 and in the sea at Okinawa Prefecture, Japan.7 In the latter studies, the fibrous adsorbent was woven into braids and anchored onto the seabed, and the average uranyl ([UO2]2+) uptake was 1.5 mg U gads−1 after soaking for 30 d.7,8Following this discovery, AO-based fibrous adsorbents with large specific surface areas have been prepared through radiation-induced grafting polymerization, using porous polyethylene fibre as the trunk material. Some have achieved nearly 3.3 mg U gads−1 in 56 d, which is approximately three times higher than that of conventional fibrous adsorbents under the same conditions.9 Because of this improvement owing to increased surface area, many scientists have focussed on nano-adsorbents for U extraction. Through surface modification, nanocarbon materials,10,11 mesoporous silica,12,13 metal–organic frameworks,14,15 and layered materials,16,17 have been developed for quick and effective U adsorption in aqueous solutions. In addition, Dai et al. have created a new mesoporous copolymer adsorbent with a large surface area and a high number of micropores. Many AO groups were subsequently grafted inside the micropores, and a U adsorption capacity of 1.99 mg U gads−1 was obtained.18,19
The majority of these nano-adsorbents, however, are inconvenient to handle in field operations because the powdery or granular adsorbents are easily washed away. In our previous work, a novel nanofibrous AO-based adsorbent was prepared via a parallel-blend electrospinning method. This approach satisfactorily resolved the run-off problem and also improved the utilization of the AO groups for adsorption.20 However, the U uptake capacity of the polyacrylamidoxime (PAO) fibrous adsorbent (obtained from polyacrylonitrile with approximately 76.8% of amidoximation degree) was only 1.85 mg U gads−1 in artificial seawater after 24 h of batch adsorption. Since the performance of other AO-based adsorbents will not significantly exceed this threshold value, other ligand groups should be introduced for more effective U extraction.
U generally exists in seawater in the very stable form of uranyl tricarbonate complex, UO2(CO3)34−.21 Its adsorption is considered a ligand exchange process between the carbonate ions and functional groups on the adsorbent, i.e., the dissociation of UO2(CO3)34− followed by the coordination of [UO2]2+ with the functional groups.22 Quantum chemistry calculations show that the reaction energies of UO2(CO3)34− with AO ligands are higher than those with AO/carboxylic (AC−) binary ligands.23 Thus, the addition of AC− groups can increase the U adsorption capacity of AO-based adsorbents by creating a synergistic effect between the two functional groups. Previously, Saito et al. prepared a hydrophilic fabric by introducing AO and AC− groups via co-graft polymerization of acrylonitrile and methacrylic acid onto a polyethylene non-woven fabric. They found that both the U adsorption rate and adsorption capacity of the developed fabric were higher than those of fabric containing only AO groups.24 Therefore, state-of-the-art AO-based adsorbents are typically prepared through radiation-induced co-grafting polymerization of acrylonitrile and (meth)acrylic acid onto various trunk fibres.9,25,26
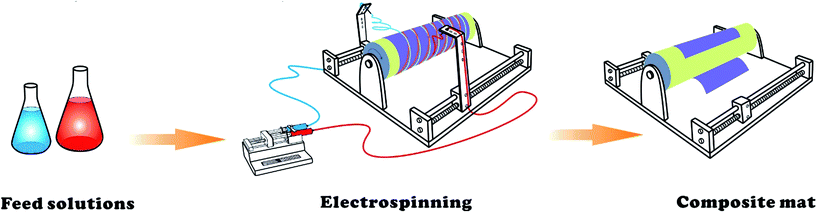
However, conventional radiation-induced graft polymerization lacks the ability of tuning the composition, grafting, conformation, and morphology of the products,27 thus hindering the preparation of high-performance adsorbents. Herein, we employ a parallel-blend electrospinning method to construct a nanofibrous adsorbent, utilizing the synergistic effect between the AO and AC− groups for U extraction from seawater. PAO (from the amidoximation of polyacrylonitrile) and polyacrylic-acid-grafted polyvinylidene fluoride (PVDF-g-PAAc) were selected as the starting materials, and they were electrospun into nanofibrous composite mats comprising an interleaving nanofibrous network created by the two material channels. The obtained composite mats possessed good porosity and hydrophilicity. Batch adsorption tests indicated that they have a significantly higher ability for [UO2]2+ adsorption than the unitary AO- or AC−-based nanofibrous adsorbents. Theoretical investigations using density functional theory (DFT) calculations including the solvation effects were also conducted to examine the mechanism behind the synergistic effect of the AO and AC− groups on the U uptake. Finally, a continuous flow-through adsorption experiment with simulated seawater (3.3 ppb U) was performed to evaluate the U extraction efficacy of the synergistic nanofibrous adsorbent.
Experimental
Chemicals and materials
Polyacrylonitrile (PAN) powder (Mw = 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was purchased from Sigma-Aldrich Co., Ltd., USA. Polyvinylidene fluoride (PVDF) powder (Mw = 420
000) was purchased from Sigma-Aldrich Co., Ltd., USA. Polyvinylidene fluoride (PVDF) powder (Mw = 420![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was purchased from Solvay Chemicals Co., Belgium. Standard solution of uranyl nitrate was purchased from Analytical Laboratory in Beijing Research Institute of Uranium Geology. Other standard solutions of co-existing metal ions, N,N-dimethyl formamide (DMF), hydroxylamine hydrochloride (NH2OH·HCl), sodium hydroxide (NaOH), acrylic acid (AAc), and other chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd., China. All chemicals were used without further purification. Deionized water was used for all experiments unless otherwise stated.
000) was purchased from Solvay Chemicals Co., Belgium. Standard solution of uranyl nitrate was purchased from Analytical Laboratory in Beijing Research Institute of Uranium Geology. Other standard solutions of co-existing metal ions, N,N-dimethyl formamide (DMF), hydroxylamine hydrochloride (NH2OH·HCl), sodium hydroxide (NaOH), acrylic acid (AAc), and other chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd., China. All chemicals were used without further purification. Deionized water was used for all experiments unless otherwise stated.
Preparation of PAO/PVDF-g-PAAc nanofibrous composite mat
The PVDF-g-PAAc was prepared via pre-irradiation graft polymerization, and PAO was obtained by amidoximation of PAN powder with NH2OH·HCl and NaOH in DMF solution, which were described in our previous reports in detail.20,28 As Scheme 1 shows, the PVDF-g-PAAc solution (with 25.6 wt% of grafting degree of PAAc, ∼0.1 g mL−1 in DMF) and PAO solution (with 76.8% of amidoximation degree, ∼0.1 g mL−1 in DMF) were loaded into two syringes and assembled in respective microinjection pumps. Then, the two nozzles were connected to a high-voltage direct current power source, and fixed on a holder that moved from left to right repeatedly while aimed at a rotary drum. The PAO and PVDF-g-PAAc nanofibers were electrospun simultaneously and interwoven uniformly into the nanofibrous composite mat. The obtained mat was immersed in deionized water for 48 h to remove inorganic salts which were introduced during amidoximation. Then, the mat was dried at 60 °C for 24 h in a vacuum oven. The parameters of the electrospinning process were set as follows: voltage, 15 kV; rotary drum speed, 500 rpm; needle-to-drum distance kept 10 cm; holder speed, 10 cm min−1; and PAO solution injection rate, 0.4 mL h−1.PVDF exhibits high chemical corrosion resistance, so it can withstand microwave digestion. Given that PAO and PAAc are easily decomposed in a microwave digestion system, the PAO content of the nanofibrous composite mats was determined by measuring the weight loss from the mats after microwave digestion and calculating the content using eqn (1).
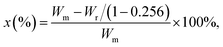 | (1) |
Preparation of artificial and simulated seawater
| U | V | Fe | Co | Ni | Cu | Zn | Pb | Mg | Ca | Na | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Actual seawater31,32 (ppb) | 3.3 | 1.5–2.5 | 1.0–2.0 | 0.05 | 1.0 | 0.6 | 4.0 | 0.03 | 1.3 × 106 | 0.4 × 106 | 1.08 × 107 |
| Artificial seawater (ppb) | 330 | 152 | 141 | 5.3 | 101 | 65 | 408 | 34.6 | 1.2 × 105 | 0.6 × 105 | 1.53 × 107 |
| Simulated seawater (ppb) | 3.6 | 1.9 | 40.6 | 0.3 | 1.1 | 5.4 | 8.2 | 31.6 | 1.2 × 105 | 0.6 × 105 | 1.53 × 107 |
| Ion species31–33 | UO2(CO3)34− | VO2(OH)32− | Fe3+ | Co2+ | Ni2+ | Cu2+ | Zn2+ | Pb2+ | Mg2+ | Ca2+ | Na+ |
| VO3− | |||||||||||
| UO2(CO3)22− | HVO42− | ||||||||||
| H2VO4− |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 2000 μs cm−1, it was consistent with the salinity of simulated seawater at 3.5 wt%. The concentration of uranium and other co-existing ions in the baysalt solution were adjusted by adding corresponding ion standard solutions, which were listed in Table 1. In which, the concentration of U, V and Ni were in accordance with that in actual seawater; Co, Cu and Zn were slightly higher than that in actual seawater still at the same level of ppb. Concentration of Fe was 10 times higher than that in actual seawater and Pb was 1000 times higher than that in actual seawater, which was due to the contamination of the baysalt obtained.
000 ± 2000 μs cm−1, it was consistent with the salinity of simulated seawater at 3.5 wt%. The concentration of uranium and other co-existing ions in the baysalt solution were adjusted by adding corresponding ion standard solutions, which were listed in Table 1. In which, the concentration of U, V and Ni were in accordance with that in actual seawater; Co, Cu and Zn were slightly higher than that in actual seawater still at the same level of ppb. Concentration of Fe was 10 times higher than that in actual seawater and Pb was 1000 times higher than that in actual seawater, which was due to the contamination of the baysalt obtained.Batch adsorption test in artificial seawater
Nanofibrous mat samples (ca. 100 mg) were immerged in 5 L artificial seawater prepared as mentioned in upper half of this section. After shaking (100 rpm) for 24 hours at room temperature, the nanofibrous mats were taken out, washed with copious water, and then dried thoroughly in a vacuum oven. The dried samples were digested with concentrated nitric acid in a MARS 6™ Microwave Digestion System from CEM Corporation, USA. The ion concentrations in the digestion solution were determined using a Perkin-Elmer Optima 8000 inductively coupled plasma-atomic emission spectrometer (ICP-AES). The metal uptake (i.e. U, V, and other co-existing metal ions) of the nanofibrous mat was evaluated according to the concentration in the digestion solution. The formula for the calculation was as follows:
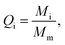 | (2) |
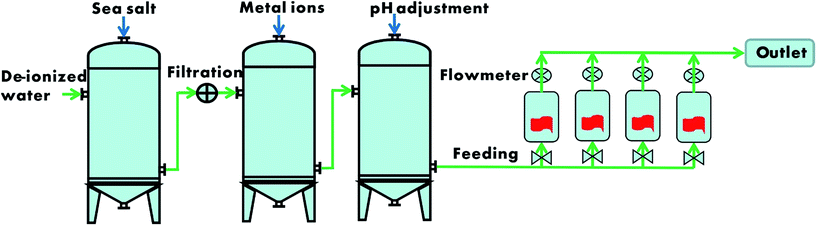
Flow-through adsorption test in simulated seawater
Flow-through experiments were carried out using a lab-scale simulated seawater adsorption system with a volumetric flow rate of 1500 L per day. The simulated seawater for continuous flow-through adsorption tests was prepared by the way described in upper section. The adsorption process was quantitatively monitored for temperature and flow rate. The nanofibrous mats (∼0.1 g) were freely dispersed in a 100 mL conical flask which was connected rubber hose as shown in Fig. S1.† Schematic diagrams of the physical layout used for adsorption exposure experiments are presented in Scheme 2. For this parallel configuration, a 12-port, all polypropylene-homo (PPH) manifold system was used. The simulated seawater was drawn from a reservoir and forced through the manifold using a pump with all-fluorine plastic components in the pump head and PPH tubing feed lines. Prior to initial use, the conical flasks, feed lines, and fittings were cleaned with a weakly acidic (5% HCl) solution and deionized water to minimize contamination. Simulated seawater adsorption testing was performed at temperature of 25 ± 2 °C, which was controlled with a thermostatic water tank before the simulated water flowed through the adsorption flasks, and at a flow rate of 20 ± 2 mL min−1 using actively pumping systems. The metal uptake (i.e. U, V, and other co-existing metal ions) of the nanofibrous mat through flow-through adsorption test was determined as the method in Batch adsorption test.General characterization
FT-IR spectra were obtained by scanning the nanofibrous mats on attenuated total reflection (ATR) module. All the FT-IR spectra were performed on a Bruker Optics TENSOR 27 FT-IR Spectrometer and were averaged over 32 scans at 4 cm−1 resolution obtained in the range of 600–4000 cm−1. The microscopic morphology of nanofibrous mats were observed with a JSM-6700F scanning electron microscope (JEOL, Japan). Samples were sputtered with 10 nm gold layer to enhance the electrical conductivity. The water static contact angle of nanofibrous mats was measured on a KSV ATTENSION Theta Optical Tensiometer. A 5 μL water drop from needle tip was stroked onto the sample surface. Shape of the droplet was recorded by a digital camera and static contact angle was calculated according to images taken by evaluation software provided from the instrument manufacturer.DFT calculations
All DFT calculations were performed using the Gaussian-09 C1 package with the B3LYP functional.34 The latter is a hybrid Hartree–Fock/DFT method that incorporates Becke's three-parameter functional (B3) with the Lee, Yang, and Parr correlation functional.35,36 Spin–orbit coupling effects were not considered during the calculations. The Stuttgart relativistic small core (RSC) 1997 effective core potential (ECP) was used for U. This potential replaces 60 inner-shell electrons of the U atom in order to incorporate scalar relativistic effects into the calculation. The remaining 32 electrons are described by the associated valence basis set.37–39 The 6-31++G* basis sets were selected for the C, N, O, and H atoms. No symmetry constraints were imposed during the structural optimizations. Frequency calculations were conducted to verify the structures at the energy minima. All the structures were fully optimized with solvation effects, which were incorporated using the conductor-like screening model (COSMO) method.40,41Results and discussion
Preparation of nanofibrous composite mat
The composite mats prepared via two-channel parallel-blend electrospinning were characterized via Fourier transform infrared (FT-IR) spectroscopy with an attenuated total reflectance (ATR) module. As shown in Fig. 1a, the peaks at 1642 and 945 cm−1 are characteristic of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– and –N–O–, respectively, in the AO group,42 and they are clearly visible in the spectra of the AC–AO-51.9 composite mat. Although the C
N– and –N–O–, respectively, in the AO group,42 and they are clearly visible in the spectra of the AC–AO-51.9 composite mat. Although the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O absorption peak of polyacrylic acid (PAAc) (1713 cm−1) is occluded by the strong peak of the –C
O absorption peak of polyacrylic acid (PAAc) (1713 cm−1) is occluded by the strong peak of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– group, the characteristic absorption bands of the –CF2 and –CH2 stretching modes (1174 and 1398 cm−1, respectively)43 are apparent in the spectra. The FT-IR results confirm that the composite mat was composed of PAO and PVDF-g-PAAc nanofibers.
N– group, the characteristic absorption bands of the –CF2 and –CH2 stretching modes (1174 and 1398 cm−1, respectively)43 are apparent in the spectra. The FT-IR results confirm that the composite mat was composed of PAO and PVDF-g-PAAc nanofibers.
Next, we examined the micromorphology of the PAO and PVDF-g-PAAc mats, along with that of the AC–AO-51.9 composite mat, using scanning electron microscopy (SEM). The PAO mat was porous, but the fibres were bonded together during the electrospinning process due to electrostatic interaction between the fibres (Fig. 1b and S2a†).20 In comparison, the PVDF-g-PAAc fibres were well separated and formed a porous microstructure (Fig. 1c and S2b†). Because of the two-component parallel-blend electrospinning, the pores in the AC–AO-51.9 composite mat were well proportioned. In contrast to the PAO mat, the fibres in AC–AO-51.9 composite mat were well separated (Fig. 1d and S2c†). The PAO nanofibers had diameters of approximately 350 nm (Fig. 1b), while those of the PVDF-g-PAAc nanofibers were 70–200 nm (Fig. 1c). In Fig. 1d, coarse, wide PAO nanofibers of approximately 350 nm diameter were interwoven with thinner PVDF-g-PAAc nanofibers of 100–200 nm diameter to form the composite mat. This micromorphology indicates that the introduction of a secondary component during the parallel-electrospinning process disrupts the electrostatic attraction between the PAO nanofibers, inducing a greater level of interweaving and a more uniform dispersal.44
Hydrophilicity and porosity of nanofibrous composite mats
The hydrophilicity and porosity of the adsorbents are important in U extraction, as these factors affect the adsorbent-seawater contact and the metal-ion uptake performance. Herein, we evaluated the hydrophilicity by water contact angle on the surfaces of various mats. According to our previous report, the pores of electrospun mats, which are formed by nanofiber interweaving, are difficult to measure using Brunauer–Emmett–Teller (BET) nitrogen adsorption and mercury porosimetry.20 This is because the large and irregularly sized pores in the electrospun mats can be deformed or crushed by the higher applied pressure during mercury porosimetry testing.45,46 Therefore, a previously reported facile assay method was used instead to measure the porosity by using the inherent density of each component and the apparent mat density.20,47 The porosity determination details of these nanofibrous composite mats can be found in our previous reports.20,29,30PAAc is a hydrophilic macromolecule that can modify hydrophobic substrates through surface grafting. The contact angle on the PVDF-g-PAAc mat (Fig. 2a, black), although lower than that of the pristine PVDF mat reported previously (not shown),20 was as large as 115°, and the water droplets did not disperse over the mat during testing. In electrospinning, nanofibers are formed during the flight of the feed-solution jets through air from the spinneret to the grounded collector, driven by the high-voltage electric field. The solvent is volatilized rapidly, and the polymeric solutes solidified into polymer wires or fibres.48 In this study, the hydrophilic PAAc segments did not migrate onto the fibre surfaces during this process. Thus, the PVDF-g-PAAc mat exhibited some hydrophobicity. However, its hydrophilicity was effectively improved when treated with an organic/water mixed solvent or a salt aqueous solution. This is because the PAAc segments can be induced to migrate to the fibre surface by H-bond or Coulomb interaction.49 On composite mats, the water contact angle decreased with increased PAO content. When the PAO content was increased to 65.7 wt% (AC–AO-65.7), the water droplets could thoroughly infiltrate the composite mat within only 1 s. In the SEM images, the fibres in the PAO mat were prone to bundling, while those in the composite mat were uniformly dispersed (Fig. 1b and d, respectively). The fibre dispersal will influence the porosity of mats with different components. Accordingly, in Fig. 2b, the PAO mat is far less porous than the composite mats, and the porosity of the latter decreases with increasing PAO content.
 | ||
| Fig. 2 (a) Hydrophilicity and (b) porosity of PVDF-g-PAAc, AC–AO-x composites, and PAO mats; x is the weight percentage of PAO in the composite mat. | ||
Batch adsorption test of composite mats and the synergistic effect
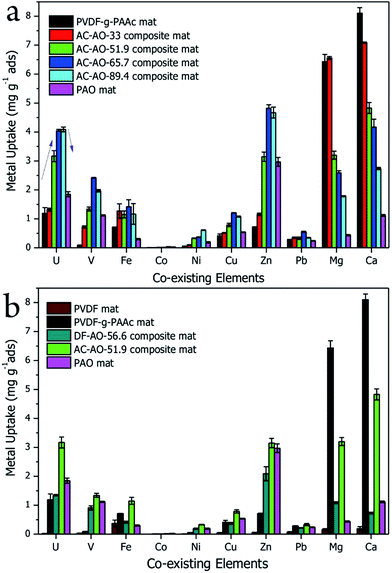
The metal uptake of the composite mats with different PAO contents were measured in a batch adsorption test conducted in artificial seawater with concentrated co-existing ions. The pH of the artificial seawater was approximately 8.0, and it contained 3.5 wt% baysalt and 10 common marine elements, namely U, V, Fe, Co, Ni, Cu, Zn, Pb, Mg, and Ca, at 100 times their respective natural concentrations in seawater (see Table 1).50 As shown in Fig. 3a, the PVDF-g-PAAc mat adsorbed all types of metal ions. Its U uptake is 1.19 mg U gads−1, which is on par with those of AO-based polyethylene non-woven or fibre adsorbents.7 Meanwhile, its V uptake was only 0.11 mg V gads−1, 1/10th of that of the PAO mat. It seems the AC-group has higher selectivity of U over V ions than AO group.51 Whereas our recent on-site adsorption tests in East China Sea, and flow-through columns adsorption tests with Sequim Bay seawater (Washington, USA) that was assisted by Marine Sciences Laboratory of Pacific Northwest National Laboratory (PNNL), we found the AC-based adsorbents captured almost zero U from seawater. This is a very weird but interesting phenomenon in the research of uranium extraction from actual seawater. We also note that the uptake of alkaline earth metals (Mg and Ca) is higher in the AC–AO-x composite mat, and the uptake capacity increases in the composite mats with decreased PAO content. Because of their relatively weak bonding with the AC− group, these cations can be desorbed easily in the solutions of weak acids (0.1 M) by immersion or elution. The U ion is subsequently eluted in 0.5 M hydrochloride solution. Therefore, the relatively large amount of adsorbed alkaline earth-metal ions is not a problem during the post-adsorption treatments for U extraction.Interestingly, the U uptake of the composite mats increases monotonically with increased PAO content up to 89.4 wt%. With 51.9 wt% PAO, the maximum U uptake is 3.17 mg U gads−1. For 89.4 wt% PAO content (AC–AO-89.4), the U uptake increases to 4.09 mg U gads−1. This value is very comparable to the highest adsorption capacity for U reported to date, in either artificial (<0.33 ppm U) or actual seawater (∼3.3 ppb U).9,52–54 This adsorption capacity is also significantly higher than that of the PAO mat, which indicates that the AC− group improves the U uptake in seawater.55 Here, we quantitatively evaluate the effect of AC− on the U uptake using the following weighted equation:
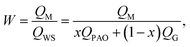 | (3) |
In order to examine this synergistic effect in composite mats with binary functional groups, we compared the metal uptake of mats containing different functional groups in artificial seawater under the same conditions. As shown in Fig. 3b, the pristine PVDF mat does not adsorb U, V, or other co-existing ions. After mixing in 56.6 wt% PAO, the composite mat (labelled DF–AO-56.6) displays an uptake of 1.35 mg U gads−1, which is lower than that of the PAO mat. While the U uptake of the PVDF-g-PAAc mat is up to 1.19 mg U gads−1, that of the AC–AO-51.9 composite mat reached 3.17 mg U gads−1, a value higher than those of both the PVDF-g-PAAc and the PAO mats. The AC–AO-51.9 and DF–AO-56.6 composite mats have similar physical characteristics (i.e., porosity, hydrophilicity, and PAO content), as shown in Table S2.† However, the U uptake of the former is approximately 2.35 times that of the latter, just because of the different PAAc content. These results further support the conclusion from Fig. 3a, that the AC− and AO groups in the composite mats have a synergistic effect on U uptake from artificial seawater containing co-existing metal ions.
DFT calculations of uranyl-absorbent interactions
To explain the mechanism of synergy between the AO− and AC− groups on U uptake, the complexation between [UO2]2+ and the AO−/AC− ligands on the adsorbents should be examined. We performed DFT calculations to assess the stable structures, bonding characteristics, and relative stabilities of the different [UO2]2+ complexes and the ligand-substituted UO2(CO3)34−. As discussed in our previous work,56 some of the relatively stable isomers of [UO2]2+ complexes with amidoximate (AO−) in the gas phase and in aqueous solution do not share the same binding motifs. Therefore, structural optimization with solvation effects should be considered here as we are more interested in the structural information in the solution phase. Shi et al. studied the interaction of [UO2]2+ with AO and AC− by adding solvation effects to the optimized structures in the gas phase.23 According to their results, the reaction UO2(CO3)34− + 3HAO → UO2(AO)3− + 3HCO3− is slightly endothermic. However, they also showed that this reaction becomes slightly exothermic when the structures are optimized directly in aqueous solution. Therefore, all the structures considered in the present calculations are fully optimized with solvation effects via the conductor-like screening model (COSMO) method.40,41The possibility of three binding motifs for [UO2]2+–AO− complexes has been suggested in a recent publication.57 All these possible binding motifs were examined in this study, and the η2 binding mode (through the AO−-ion N–O bond) was determined as the most favourable for [UO2]2+–AO− complexes. AC− can bind to [UO2]2+ through the monodentate and bidentate binding motifs. All optimized structures for [UO2]2+ complexes with AO−, AC−, and mixed AO−/AC− in aqueous solution are displayed in Fig. S3.† Selected bond distances in [UO2]2+ complexes are shown in Table S3.† The bond lengths between U and the axial O atoms in [UO2(CO3)2(AO)]3−, [UO2(CO3)(AO)2]2−, and [UO2(AO)3]− are comparable, implying that the AO− ligands have comparable affinities for [UO2]2+ to the carbonate ligands. These bond lengths between U and the axial O atoms for [UO2(CO3)2(AC)]3−, [UO2(CO3)(AC)2]2−, and [UO2(AC)3]− decrease in this order, implying that the AC− ligands exhibit weaker affinities for [UO2]2+ than the carbonate ligands. The U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond lengths in [UO2(AO)2(AC)]− and [UO2(CO3)(AO)(AC)]2− are comparable and both are longer than that of [UO2(AO)(AC)2]−. These results indicate that the electron-donating abilities of AO− and CO32− are comparable and stronger than that of AC−. The distances between the U and ligand atoms are in the order of U–O(AO−) < U–N(AO−) < U–O(AC−). Thus, U–O(AO−) plays the most important role in the interaction between [UO2]2+ and its equatorial ligands, followed by U–N(AO−) and U–O(AC−).
O bond lengths in [UO2(AO)2(AC)]− and [UO2(CO3)(AO)(AC)]2− are comparable and both are longer than that of [UO2(AO)(AC)2]−. These results indicate that the electron-donating abilities of AO− and CO32− are comparable and stronger than that of AC−. The distances between the U and ligand atoms are in the order of U–O(AO−) < U–N(AO−) < U–O(AC−). Thus, U–O(AO−) plays the most important role in the interaction between [UO2]2+ and its equatorial ligands, followed by U–N(AO−) and U–O(AC−).
To gain more insight into the synergistic effect between AO− and AC− in U adsorption, natural bond orbital (NBO) analysis was conducted for [UO2]2+ complexes with different AO−/AC− ligands. As shown in Table 2, the natural charge on U increases when AC− replaces AO− in the complex. This indicates that the electron-donating ability of AO− is stronger than that of AC−, which is in accordance with the U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond lengths mentioned above. The Wiberg bond indices (WBIs)58 of the bonds between U and the ligand atoms are in the same order as the bond strength, i.e., U–O(AO−) > U–N(AO−) > U–O(AC−). The hybrid σ(U–O(AO−)), σ(U–O(AC−)), and σ(U–N(AO−)) orbitals are presented in Table 3. Along the columns, when AO− is replaced by AC− stepwise in the complex, the U character of the σ(U–O(AO−)) and σ(U–N(AO−)) orbitals first increases and then decreases, while the U character of σ(U–O(AC−)) increases. The σ(U–O(AC−)) orbital in [UO2(AO)2(AC)]− does not exist; instead, there are only lone O pairs. Our previous studies have confirmed that the higher covalent character of U–O(AO−) and the greater contribution of the U 5f/6d orbitals to U–O(AO−) bonding may be responsible for the excellent binding ability of AO−.59,60 Therefore, the AC− group involvement in [UO2]2+ complexation can slightly increase the U 6d/5f orbital contribution to the hybrid σ(U–O(AO−)) and σ(U–N(AO−)) orbitals. This may be one of the causes of the synergistic effect between AO− and AC− during U adsorption.
O bond lengths mentioned above. The Wiberg bond indices (WBIs)58 of the bonds between U and the ligand atoms are in the same order as the bond strength, i.e., U–O(AO−) > U–N(AO−) > U–O(AC−). The hybrid σ(U–O(AO−)), σ(U–O(AC−)), and σ(U–N(AO−)) orbitals are presented in Table 3. Along the columns, when AO− is replaced by AC− stepwise in the complex, the U character of the σ(U–O(AO−)) and σ(U–N(AO−)) orbitals first increases and then decreases, while the U character of σ(U–O(AC−)) increases. The σ(U–O(AC−)) orbital in [UO2(AO)2(AC)]− does not exist; instead, there are only lone O pairs. Our previous studies have confirmed that the higher covalent character of U–O(AO−) and the greater contribution of the U 5f/6d orbitals to U–O(AO−) bonding may be responsible for the excellent binding ability of AO−.59,60 Therefore, the AC− group involvement in [UO2]2+ complexation can slightly increase the U 6d/5f orbital contribution to the hybrid σ(U–O(AO−)) and σ(U–N(AO−)) orbitals. This may be one of the causes of the synergistic effect between AO− and AC− during U adsorption.
| Species | Q(U) | Q(Oaxial) | U–O(AO−) | U–N(AO−) | U–O(AC−) |
|---|---|---|---|---|---|
| [UO2(AO)3]− | 0.968 | −0.542 | 0.666 | 0.488 | |
| [UO2(AO)2(AC)]− | 1.096 | −0.532 | 0.699 | 0.510 | 0.404 |
| [UO2(AO)(AC)2]− | 1.238 | −0.520 | 0.746 | 0.530 | 0.419 |
| [UO2(AC)3]− | 1.399 | −0.504 | 0.443 |
| σ(U–O(AO−)) | σ(U–O(AC−)) | σ(U–N(AO−)) | |
|---|---|---|---|
| [UO2(AO)3]− | 13%(41% 6d, 25% 5f) | 13%(34% 6d, 20% 5f) | |
| [UO2(AO)2(AC)]− | 14%(43% 6d, 26% 5f) | — | 14%(37% 6d, 27% 5f) |
| [UO2(AO)(AC)2]− | 14%(41% 6d, 28% 5f) | 10%(38% 6d, 21% 5f) | 14%(36% 6d, 21% 5f) |
| [UO2(AC)3]− | 10%(39% 6d, 28% 5f) |
To evaluate the relative stabilities of these complexes, we studied the [UO2]2+-ligand complexation reactions in aqueous solutions. As shown in Table 4a, although the binding free energies for [UO2]2+ complexes with 1–3 AO− ligands are all comparable, the complex becomes slightly more stable when AO− replaces CO32−. In contrast, the [UO2]2+ complex becomes less stable when AC− replaces CO32−. Thus, the affinity of AO− for [UO2]2+ is comparable to that of CO32−, while that of AC− is significantly lower. These results also agree well with the U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond lengths and the NBO results.
O bond lengths and the NBO results.
| Reaction | ΔG | |
|---|---|---|
| L = AO− | L = AC− | |
| (a) | ||
| [UO2(H2O)5]2+ + L− + 2CO32− → UO2L(CO3)23− + 5H2O | −106.3 | −93.3 |
| [UO2(H2O)5]2+ + 2L− + CO32− → UO2(L)2(CO3)2− + 5H2O | −106.9 | −80.3 |
| [UO2(H2O)5]2+ + 3L− → UO2(L)3− + 5H2O | −107.5 | −64.1 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| (b) | ||
| UO2(CO3)34− + HL → UO2L(CO3)23− + HCO3− | −1.3 | −7.1 |
| UO2(CO3)34− + 2HL → UO2(L)2(CO3)2− + 2HCO3− | 0.4 | −11.4 |
| UO2(CO3)34− + 3HL → UO2(L)3− + 3HCO3− | 4.0 | −12.5 |
| UO2(CO3)34− + HAO + HAC → UO2(AO)(AC)(CO3)2− + 2HCO3− | −6.5 | |
| UO2(CO3)34− + 2HAO + HAC → UO2(AO)2(AC)− + 3HCO3− | −3.8 | |
| UO2(CO3)34− + HAO + 2HAC → UO2(AO)(AC)2− + 3HCO3− | −9.9 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| (c) | ||
| HAO + H2O → AO− + H3O+ | 54.9 | |
| HAC + H2O → AC− + H3O+ | 35.3 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| (d) | ||
| UO2(CO3)34− + 5H2O → [UO2(H2O)5]2+ + CO32− | 103.4 | |
| UO2(CO3)34− + 3H3O+ + 2H2O → [UO2(H2O)5]2+ + 3HCO3− | −54.3 | |
Finally, we studied the substitution reactions of UO2(CO3)34− by HAO or HAC to form [UO2]2+ complexes in aqueous solution. As shown in Table 4b, the changes in the free energy (ΔG) for the substitution reactions leading to [UO2]2+–AC− complexes are more negative than those leading to [UO2]2+–AO− complexes; therefore, the former are thermodynamically more favourable than the latter. Since AO− was found to interact more strongly with [UO2]2+ than AC−, it seems paradoxical that the reaction of UO2(CO3)34− with HAC is thermodynamically more favourable than that with HAO. This behaviour is most likely due to the higher dissociation constant of HAC than HAO (Table 4c), because the produced H+ strongly favours the dissociation of UO2(CO3)34− (Table 4d). This may be another source of the observed synergistic effect between AO− and AC− during U adsorption.
Flow-through adsorption test of nanofibrous composite mat
Based on the outstanding U uptake displayed by the AC–AO-x composite mats with concentrated co-existing ions, we conducted another evaluation of their adsorption performance in simulated seawater with a U concentration of approximately 3.3 ppb, accompanied by co-existing metal ions at their natural concentrations (see details in Table 1). The basic process of the flow-through adsorption test is shown in Scheme 2. Fig. 4a shows that the uptake values of most metal ions in the AC–AO-65.7 nanofibrous mat increased with time, except those of Ca and Mg which were almost constant throughout the adsorption process. The latter indicates rapid equilibrium of the ionization of carboxylic acid and the ion-exchanging reactions involving AC− and Ca/Mg ions in the simulated seawater. Further, it is apparent that the large amount of alkaline earth ions in simulated seawater is not an obvious hindrance to the U extraction onto adsorbent. In Fig. 4b, the composite mats kept adsorbing U throughout the experiment (30 d). The final U uptake reached 2.86 mg U gads−1, which is very comparable, even superior to the U uptake from flow-through adsorption test in simulated or filtered actual seawater of the various advanced fibrous adsorbents from Oak Ridge National Laboratory (ORNL) and other institutes within same soaking days.61–63 More significantly, U uptake of the composite mats did not reach equilibrium within 30 d soaking. The initial U uptake rate was 3.5 mg U gads−1 d−1. After 5 d, a constant rate of 0.1 mg U gads−1 d−1 was maintained. Both values are significantly higher than the reported rates for unitary AO-based porous nano-adsorbents (∼2.7 and ∼0.03 mg U gads−1 d−1, respectively),18 as well as those for porous polyethylene fibrous AO-based adsorbents (0.19 and ∼0.02 mg U gads−1 d−1, respectively).9 Therefore, it is believable that the maximum U uptake ability of the composite mat must be larger than 2.86 mg U gads−1 when exposed for a longer period, at least higher than 3.3 mg U gads−1.9 | ||
| Fig. 4 (a) Metal uptake and (b) U uptake of the AC–AO-65.7 nanofibrous mat in simulated seawater over 30 d. | ||
Conclusions
We have demonstrated a highly effective nanofibrous composite mat containing both AO and AC− groups for U adsorption in simulated seawater with 3.3 ppb U, using the synergistic effect between the AO and AC− groups. Compared to unitary AO-based adsorbents, this new nanofibrous adsorbent exhibited greater U adsorption capacity in artificial seawater with concentrated co-existing metal ions. It also displays higher U uptake efficiency and higher U uptake rate in a flow-through adsorption test using simulated seawater with 3.3 ppb U. The results of the flow-through test indicate the feasibility of our nanofibrous composite mat for U extraction from seawater. According to our theoretical DFT calculations, the AC− group involvement in the [UO2]2+ complexation moderately increases the contribution of the U 6d/5f orbitals to U–AO binding. Furthermore, the H ions produced via HAC dissociation strongly facilitate the dissociation of the UO2(CO3)34− complex and increase the [UO2]2+–AO binding efficiency. The successful fabrication of composite mats containing binary functional groups via parallel-blend electrospinning suggests a versatile, highly scalable method for preparing multifunctional nanofibrous materials for metal recycling, water purification, and other separation applications. As a proof of concept, in this work we developed the AO/AC-based nanofibrous composite mats as a model synergistic U adsorbent. In short, the results presented here will facilitate the development of new types of nanofibrous functional adsorbents for a wide range of applications.Acknowledgements
We greatly appreciate the financial supports from the “Strategic Priority Research Program” of the Chinese Academy of Sciences (Grant No. XDA02040300), and the National Natural Science Foundation of China (Grants No. 11305248 and 11305241).Notes and references
- IAEA and OECD/NEA, International Atomic Energy Agency (IAEA) & Nuclear Energy Agency (NEA) of the Organisation for Economic Co-operation and Development (OECD), 2014 Search PubMed.
- U. Bardi, Sustainability, 2010, 2, 980–992 CrossRef CAS.
- D. S. Sholl and R. P. Lively, Nature, 2016, 532, 435–437 CrossRef PubMed.
- R. V. Davies, J. Kennedy, R. W. McIlroy, R. Spence and K. M. Hill, Nature, 1964, 203, 1110–1115 CrossRef.
- Y. Koyanaka, J. Nucl. Sci. Technol., 1970, 7, 426–427 CrossRef CAS.
- N. Seko, A. Katakai, S. Hasegawa, M. Tamada, N. Kasai, H. Takeda, T. Sugo and K. Saito, Nucl. Technol., 2003, 144, 274–278 CAS.
- M. Tamada, Japan Atomic Energy Agency, Vienna, 2009 Search PubMed.
- E. Schneider and D. Sachde, Sci. Global Secur., 2013, 21, 134–163 CrossRef.
- J. Kim, C. Tsouris, Y. Oyola, C. J. Janke, R. T. Mayes, S. Dai, G. Gill, L.-J. Kuo, J. Wood, K.-Y. Choe, E. Schneider and H. Lindner, Ind. Eng. Chem. Res., 2014, 53, 6076–6083 CrossRef CAS.
- Z. Li, F. Chen, L. Yuan, Y. Liu, Y. Zhao, Z. Chai and W. Shi, Chem. Eng. J., 2012, 210, 539–546 CrossRef CAS.
- Y. Sun, D. Shao, C. Chen, S. Yang and X. Wang, Environ. Sci. Technol., 2013, 47, 9904–9910 CrossRef CAS PubMed.
- L.-Y. Yuan, Y.-L. Liu, W.-Q. Shi, Y.-L. Lv, J.-H. Lan, Y.-L. Zhao and Z.-F. Chai, Dalton Trans., 2011, 40, 7446–7453 RSC.
- W. Zhang, G. Ye and J. Chen, J. Mater. Chem. A, 2013, 1, 12706–12709 CAS.
- M. Carboni, C. W. Abney, S. Liu and W. Lin, Chem. Sci., 2013, 4, 2396–2402 RSC.
- Z.-Q. Bai, L.-Y. Yuan, L. Zhu, Z.-R. Liu, S.-Q. Chu, L.-R. Zheng, J. Zhang, Z.-F. Chai and W.-Q. Shi, J. Mater. Chem. A, 2015, 3, 525–534 CAS.
- M. J. Manos and M. G. Kanatzidis, J. Am. Chem. Soc., 2012, 134, 16441–16446 CrossRef CAS PubMed.
- S. L. Ma, L. Huang, L. J. Ma, Y. Shim, S. M. Islam, P. L. Wang, L. D. Zhao, S. C. Wang, G. B. Sun, X. J. Yang and M. G. Kanatzidis, J. Am. Chem. Soc., 2015, 137, 3670–3677 CrossRef CAS PubMed.
- Y. Yue, R. T. Mayes, J. Kim, P. F. Fulvio, X.-G. Sun, C. Tsouris, J. Chen, S. Brown and S. Dai, Angew. Chem., Int. Ed., 2013, 52, 13458–13462 CrossRef CAS PubMed.
- Y. Lu, Nat. Chem., 2014, 6, 175–177 CrossRef CAS PubMed.
- S. Xie, X. Liu, B. Zhang, H. Ma, C. Ling, M. Yu, L. Li and J. Li, J. Mater. Chem. A, 2015, 3, 2552–2558 CAS.
- F. Endrizzi and L. Rao, Chem.–Eur. J., 2014, 20, 14499–14506 CrossRef CAS PubMed.
- T. Hirotsu, S. Katoh, K. Sugasaka, M. Seno and T. Itagaki, J. Chem. Soc., Dalton Trans., 1986, 1983–1986 RSC.
- C.-Z. Wang, J.-H. Lan, Q.-Y. Wu, Q. Luo, Y.-L. Zhao, X.-K. Wang, Z.-F. Chai and W.-Q. Shi, Inorg. Chem., 2014, 53, 9466–9476 CrossRef CAS PubMed.
- T. Kawai, K. Saito, K. Sugita, A. Katakai, N. Seko, T. Sugo, J.-i. Kanno and T. Kawakami, Ind. Eng. Chem. Res., 2000, 39, 2910–2915 CrossRef CAS.
- Z. Xing, J. Hu, M. Wang, W. Zhang, S. Li, Q. Gao and G. Wu, Sci. China: Chem., 2013, 56, 1504–1509 CrossRef CAS.
- J. Kim, Y. Oyola, C. Tsouris, C. R. Hexel, R. T. Mayes, C. J. Janke and S. Dai, Ind. Eng. Chem. Res., 2013, 52, 9433–9440 CrossRef CAS.
- T. Saito, S. Brown, S. Chatterjee, J. Kim, C. Tsouris, R. T. Mayes, L.-J. Kuo, G. Gill, Y. Oyola, C. J. Janke and S. Dai, J. Mater. Chem. A, 2014, 2, 14674–14681 CAS.
- B. Deng, M. Yu, X. Yang, B. Zhang, L. Li, L. Xie, J. Li and X. Lu, J. Membr. Sci., 2010, 350, 252–258 CrossRef CAS.
- Y. Ma, B. Zhang, H. Ma, M. Yu, L. Li and J. Li, RSC Adv., 2016, 6, 30739–30746 RSC.
- Y. Ma, B. Zhang, H. Ma, M. Yu, L. Li and J. Li, Sci. China Mater., 2016, 59, 38–50 CrossRef.
- H. Sodaye, S. Nisan, C. Poletiko, S. Prabhakar and P. K. Tewari, Desalination, 2009, 235, 9–32 CrossRef CAS.
- D. Wang and S. A. Sañudo-Wilhelmy, Mar. Chem., 2008, 112, 72–80 CrossRef CAS.
- K. K. Turekian, Handbook of Geochemistry, Springer-Verlag, Berlin, 1969 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian, Inc., Wallingford CT, 2009.
- A. D. Becke, Phys. Rev. A, 1988, 38, 3098–3100 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS.
- W. Küchle, M. Dolg, H. Stoll and H. Preuss, Mol. Phys., 1991, 74, 1245–1263 CrossRef.
- M. Dolg, H. Stoll, H. Preuss and R. M. Pitzer, J. Phys. Chem., 1993, 97, 5852–5859 CrossRef CAS.
- W. Küchle, M. Dolg, H. Stoll and H. Preuss, J. Chem. Phys., 1994, 100, 7535–7542 CrossRef.
- A. Klamt and G. Schuurmann, J. Chem. Soc., Perkin Trans. 2, 1993, 799–805 RSC.
- C. C. Pye and T. Ziegler, Theor. Chem. Acc., 1999, 101, 396–408 CrossRef CAS.
- J. Hu, H. Ma, Z. Xing, X. Liu, L. Xu, R. Li, C. Lin, M. Wang, J. Li and G. Wu, Ind. Eng. Chem. Res., 2016, 55, 4118–4124 CrossRef CAS.
- B. Deng, Y. Yu, B. Zhang, X. Yang, L. Li, M. Yu and J. Li, Radiat. Phys. Chem., 2011, 80, 159–163 CrossRef CAS.
- D. Christopher, W. Xianyan, A. S. Lynne and K. Jayant, in Polymeric Nanofibers, American Chemical Society, 2006, pp. 137–148 Search PubMed.
- G. C. Rutledge, J. L. Lowery and C. L. Pai, J. Eng. Fibers Fabr., 2009, 4, 1–13 CAS.
- J. L. Lowery, N. Datta and G. C. Rutledge, Biomaterials, 2010, 31, 491–504 CrossRef CAS PubMed.
- X. Zhu, W. Cui, X. Li and Y. Jin, Biomacromolecules, 2008, 9, 1795–1801 CrossRef CAS PubMed.
- A. Greiner and J. H. Wendorff, Angew. Chem., Int. Ed., 2007, 46, 5670–5703 CrossRef CAS PubMed.
- W. Zhang, Y. Zhu, X. Liu, D. Wang, J. Li, L. Jiang and J. Jin, Angew. Chem., Int. Ed., 2014, 53, 856–860 CrossRef CAS PubMed.
- J. Kim, C. Tsouris, R. T. Mayes, Y. Oyola, T. Saito, C. J. Janke, S. Dai, E. Schneider and D. Sachde, Sep. Sci. Technol., 2012, 48, 367–387 CrossRef.
- S. P. Kelley, P. S. Barber, P. H. K. Mullins and R. D. Rogers, Chem. Commun., 2014, 50, 12504–12507 RSC.
- G. A. Gill, L.-J. Kuo, C. J. Janke, J. Park, R. T. Jeters, G. T. Bonheyo, H.-B. Pan, C. Wai, T. Khangaonkar, L. Bianucci, J. R. Wood, M. G. Warner, S. Peterson, D. G. Abrecht, R. T. Mayes, C. Tsouris, Y. Oyola, J. E. Strivens, N. J. Schlafer, R. S. Addleman, W. Chouyyok, S. Das, J. Kim, K. Buesseler, C. Breier and E. D'Alessandro, Ind. Eng. Chem. Res., 2016, 55, 4264–4277 CrossRef CAS.
- Y. Oyola, C. J. Janke and S. Dai, Ind. Eng. Chem. Res., 2016, 55, 4149–4160 CrossRef CAS.
- S. Das, S. Brown, R. T. Mayes, C. J. Janke, C. Tsouris, L. J. Kuo, G. Gill and S. Dai, Chem. Eng. J., 2016, 298, 125–135 CrossRef CAS.
- S.-H. Choi and Y. C. Nho, Radiat. Phys. Chem., 2000, 57, 187–193 CrossRef CAS.
- X. Guo, L. Huang, C. Li, J. Hu, G. Wu and P. Huai, Phys. Chem. Chem. Phys., 2015, 17, 14662–14673 RSC.
- S. Vukovic, L. A. Watson, S. O. Kang, R. Custelcean and B. P. Hay, Inorg. Chem., 2012, 51, 3855–3859 CrossRef CAS PubMed.
- K. B. Wiberg, Tetrahedron, 1968, 24, 1083–1096 CrossRef CAS.
- X. Guo, X.-G. Xiong, C. Li, H. Gong, P. Huai, J. Hu, C. Jin, L. Huang and G. Wu, Inorg. Chim. Acta, 2016, 441, 117–125 CrossRef CAS.
- L. Zhang, J. Su, S. Yang, X. Guo, Y. Jia, N. Chen, J. Zhou, S. Zhang, S. Wang, J. Li, J. Li, G. Wu and J.-Q. Wang, Ind. Eng. Chem. Res., 2016, 55, 4224–4230 CrossRef CAS.
- S. Das, Y. Oyola, R. T. Mayes, C. J. Janke, L. J. Kuo, G. Gill, J. R. Wood and S. Dai, Ind. Eng. Chem. Res., 2016, 55, 4110–4117 CrossRef CAS.
- S. Das, Y. Oyola, R. T. Mayes, C. J. Janke, L. J. Kuo, G. Gill, J. R. Wood and S. Dai, Ind. Eng. Chem. Res., 2016, 55, 4103–4109 CrossRef CAS.
- S. Brown, Y. Yue, L.-J. Kuo, N. Mehio, M. Li, G. Gill, C. Tsouris, R. T. Mayes, T. Saito and S. Dai, Ind. Eng. Chem. Res., 2016, 55, 4139–4148 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra18785d |
| ‡ Dr B. Zhang and Dr X. Guo are co-first authors of this work. |
| This journal is © The Royal Society of Chemistry 2016 |