Large-area 2D microgel colloidal crystals fabricated via benzophenone-based photochemical reaction†
Junying Wenga,
Xiaoyun Lia,
Ying Guan*a,
X. X. Zhub and
Yongjun Zhang *a
*a
aKey Laboratory of Functional Polymer Materials, State Key Laboratory of Medicinal Chemical Biology, The Co-Innovation Center of Chemistry and Chemical Engineering of Tianjin, Institute of Polymer Chemistry, College of Chemistry, Nankai University, Tianjin 300071, China. E-mail: yingguan@nankai.edu.cn; yongjunzhang@nankai.edu.cn
bDepartment of Chemistry, Université de Montréal, C. P. 6128, Succursale Centre-ville, Montreal, QC H3C 3J7, Canada
First published on 22nd August 2016
Abstract
We report a simple method to fabricate high quality, large area 2D colloidal crystals (CCs) of a soft colloid, poly(N-isopropylacrylamide) (PNIPAM) microgel. The microgel spheres were first assembled into a 3D CC. The first 111 plane of the 3D CC close to the substrate was then fixed in situ onto the substrate via a photochemical reaction of the benzophenone moieties immobilized on the substrate surface. The resulting 2D microgel arrays exhibit a high degree of ordering. The interparticle distance in the 2D array can be tuned by the concentration of the microgel dispersion. Increasing the concentration of the microgel dispersion will reduce the interparticle distance of the resulting 2D array. Because large area 3D microgel CCs can be facilely fabricated, this method allows for the fabrication of large area 2D microgel CCs. In addition, the method allows the 2D CCs to be fabricated on nonplanar substrates.
Introduction
Monodisperse colloidal particles can be assembled into 3D colloidal crystals (CCs) and also 2D CCs.1–3 Like 3D CCs,4 2D CCs have also found important applications, including as photonic crystals,2 in surface patterns,5 sensors,6–9 and nanolithography.10,11 Therefore numerous 2D CCs have been prepared by different methods, for example, spin-coating,12–14 interfacial assembly,6,7 and convective assembly.1,15,16 These CCs are usually assembled from hard colloids, such as polystyrene (PS),6,8,9,16–20 poly(methyl methacrylate) (PMMA),21,22 and SiO2.13,16,23 For example, Kraus et al.16 assembled PS spheres into 2D CCs via convective assembly and carefully studied the crystallization mechanisms. Jiang et al.13,23 fabricated non-close-packed 2D CC of silica particles via a spin-coating procedure. Recently Meng et al.22 reported the preparation of 2D CC of PMMA colloids by assembling the colloids on an air–water surface.In contrast, studies on 2D CCs of soft hydrogel colloids, for example poly(N-isopropylacrylamide) (PNIPAM) microgel spheres, is relatively sparse.24 Like hard colloids, monodisperse PNIPAM microgel spheres can be facilely synthesized via precipitation polymerization.25–27 Different from hard spheres, however, PNIPAM microgel spheres could respond to external stimuli and thus change their size and properties.26,27 The stimuli-responsivity makes 2D microgel CCs promising for many potential applications. For example, it was found that the attachment and growth of cells seeded on 2D microgel CC can be controlled by temperature.24 Previously 2D microgel CCs were fabricated via dip coating,28,29 solvent evaporation,27,30–33 or interfacial assembly.11,34,35 It was revealed that these soft colloids are more difficult to be assembled into ordered 2D arrays than hard particles, because the 2D ordering is easy to be lost upon drying or transferring, due to the soft nature of the spheres.24
Like 2D CCs of hard colloids, there are still some severe limitations for the existing 2D microgel CCs. One problem is that their size is usually small.6 For example, 2D microgel CCs were previously fabricated by air-drying a diluted microgel dispersion.30 Although the method is extremely simple, the resulting colored array has a small size of only 0.36 cm2 and the ordering is relatively low. Another problem is that most of the methods, for example the spin coating method,11 only allow for the use of planar substrates. Some methods, for example the one developed by Isa et al.34 requires certain specific equipment (Langmuir trough). To overcome these limitations, we proposed a facile method to fabricate 2D CC of microgel spheres (Scheme 1). The microgel spheres were first assembled into 3D CCs. Then the first 111 plane was fixed in situ onto the substrate via a photochemical reaction. Because large area, high quality 3D microgel CCs can be facilely fabricated,36,37 this method allows for fabricating large area, high quality 2D microgel CCs. In addition, non-planar substrates can also be used. We previously used thiol–ene reaction for the fixation purpose.38 This reaction requires not only the modification of the substrate, but also the microgel spheres. Here a benzophenone-based photochemical reaction was selected to fix the microgel spheres on the substrate.39–41 In this method the modification of the microgel is no longer required, allowing large area 2D CCs to be fabricated from the pristine microgel spheres.
 | ||
| Scheme 1 Fabrication of 2D microgel CC by first assembly of 3D microgel CC followed by in situ fixing of the first 111 plane onto the substrate via a BP-based photochemical reaction. | ||
Experimental
Materials
N-Isopropylacrylamide (NIPAM) and N,N′-methylenebis(acrylamide) (BIS) were purchased from Alfa Aesar. Acrylic acid (AA) and 3-aminopropyltriethoxysilane (APTES) were purchased from Acros. 4-Aminobenzophenone (ABP), hexahydrophthalic anhydride, N-(3-dimethylaminopropyl)-N-ethyl-carbodiimide hydrochloride (EDC), potassium persulfate (KPS), N-hydroxysuccinimide (NHS) were purchased from local providers. NIPAM was purified by recrystallization from hexane/acetone mixture and dried in a vacuum. AA was distilled under reduced pressure.Microgel synthesis
A series of PNIPAM microgels with different sizes were synthesized via free-radical precipitation polymerization. The 880 nm microgel (hydrodynamic diameter (Dh) = 880 nm at 20 °C) was synthesized as follows. First NIPAM, BIS, and AA were dissolved in 95 mL of water. The total amount of the monomers was 0.015 mol, and their molar ratio, NIPAM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AA
AA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BIS, was 88.5
BIS, was 88.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5. The solution was purged with nitrogen and heated to 70 °C. After 1 h, 5 mL of 0.06 M KPS solution was added to initiate the polymerization. The reaction was allowed to process for 4 h. The resulting microgels were purified by three successive centrifugation (12
1.5. The solution was purged with nitrogen and heated to 70 °C. After 1 h, 5 mL of 0.06 M KPS solution was added to initiate the polymerization. The reaction was allowed to process for 4 h. The resulting microgels were purified by three successive centrifugation (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 1 h) followed by decantation and redispersion in water. To synthesize microgels with bigger sizes, when the above reaction proceeded for 4 h, a 100 mL of preheated shell solution was added. The recipes of the shell solution were shown in Table 1. The reaction was allowed to proceed for additional 4 h. The microgels were again purified by centrifugation followed by decantation and redispersion in water. The microgels were named according to their Dh at 20 °C.
000g for 1 h) followed by decantation and redispersion in water. To synthesize microgels with bigger sizes, when the above reaction proceeded for 4 h, a 100 mL of preheated shell solution was added. The recipes of the shell solution were shown in Table 1. The reaction was allowed to proceed for additional 4 h. The microgels were again purified by centrifugation followed by decantation and redispersion in water. The microgels were named according to their Dh at 20 °C.
| Sample name | Total amount of the monomer (mol) | Molar ratio NIPAM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AA AA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BIS BIS |
KPS (M) | Dh at 20 °C (nm) |
|---|---|---|---|---|
| 1450 nm microgel | 0.015 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0.003 | 1450 |
| 1550 nm microgel | 0.015 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.003 | 1550 |
| 1800 nm microgel | 0.015 | 88.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
0.003 | 1800 |
Synthesis of silane coupling agent with a carboxylic acid end functional group42,43
Hexahydrophthalic anhydride (326 mg, 1 mmol) was dissolved in 20 mL of anhydrous toluene, to which 23 mL of APTES (1.1 equiv.) was added dropwise. The mixture was stirred at room temperature for 2 h. The solid product was filtered, and recrystallized from petroleum ether/toluene mixture. Yield: 80%. 1H NMR (D2O): δ 0.6–0.78 (t, 2H), 1.16–1.32 (t, 9H), 1.41–1.68 (m, 6H), 1.73–1.85 (m, 2H), 1.87–2.08 (m, 2H), 2.77–2.85 (t, 1H, J = 3.1 Hz), 2.87–2.95 (t, 1H, J = 2.0 Hz), 3.15–3.3 (m, 2H), 3.6–3.74 (q, 6H).Surface modification of quartz slides
Quartz slides with size of 25 × 45 × 1 mm, unless otherwise specified, were used as substrate for the fabrication of 2D CC. They were cleaned with freshly prepared piranha solution (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v H2O2–H2SO4 mixture) (caution: this solution is extremely corrosive!) for 6 h, rinsed with deionized water thoroughly, and dried in a stream of nitrogen. To introduce surface carboxylic acid groups, the cleaned substrate were immersed in a 15 mM ethanolic solution of the silane coupling agent synthesized above for 12 h at room temperature, rinsed with ethanol extensively, and dried in a stream of nitrogen. To activate the surface carboxylic acid groups, the substrate was immersed in pH 6 phosphate buffer containing EDC (10 mM) and NHS (5 mM) at 4 °C for 2 h, rinsed extensively in deionized water and dried in a stream of nitrogen.44 The NHS ester-functionalized substrate was then immersed in 20 mM ABP in DMSO at room temperature for 12 h, washed with DMSO extensively, and dried in a stream of nitrogen.41
7 v/v H2O2–H2SO4 mixture) (caution: this solution is extremely corrosive!) for 6 h, rinsed with deionized water thoroughly, and dried in a stream of nitrogen. To introduce surface carboxylic acid groups, the cleaned substrate were immersed in a 15 mM ethanolic solution of the silane coupling agent synthesized above for 12 h at room temperature, rinsed with ethanol extensively, and dried in a stream of nitrogen. To activate the surface carboxylic acid groups, the substrate was immersed in pH 6 phosphate buffer containing EDC (10 mM) and NHS (5 mM) at 4 °C for 2 h, rinsed extensively in deionized water and dried in a stream of nitrogen.44 The NHS ester-functionalized substrate was then immersed in 20 mM ABP in DMSO at room temperature for 12 h, washed with DMSO extensively, and dried in a stream of nitrogen.41
Determination of surface carboxylic acid groups
The amount of carboxylic acid groups immobilized on the surface of the substrate was determined by a colorimetric method based on Toluidine Blue O staining, assuming the molar ratio between carboxylic acid groups and the adsorbed dye to be unity.41,45 The substrate was first immersed in a 0.5 mM Toluidine Blue O solution (pH 10) at 30 °C for 6 h, and thoroughly washed with pH 10 NaOH solution to remove any dye nonspecifically adhered to the surface. The substrate was then soaked in a 50% acetic acid solution to desorb the dye molecules from the surface. The amount of the released dye was then determined from the optical density (OD) of the solution at 633 nm.Fabrication of 2D microgel arrays
The microgel dispersions were first concentrated by centrifugation. They were then loaded into a cell composed of two quartz slides (one of them was surface-modified) separated by 2 layers of Parafilm. After one day aging at 20 °C the microgel particles self-assembled gradually into a highly ordered crystalline structure. The crystallized samples were then irradiated with UV light (λ > 340 nm) for 30 min at an integral light intensity of 100 mW cm−2. Finally the reaction cell was opened and the substrate was rinsed in flowing DI water to remove microgel particles not covalently bonded to the substrate.Characterization
The size and size distribution of microgel particles were determined by dynamic light scattering with a Brookhaven 90Plus laser particle size analyzer. The measurements were all carried out at a scattering angle of 90°. The sample temperature was controlled with a build-in Peltier temperature controller. UV-vis absorption spectra were measured on a TU 1810PC UV-vis spectrophotometer (Purkinje General, China). 1H NMR spectra were measured on a Varian UNITY-plus 400 NMR spectrometer using D2O as solvent. Atomic force microscopy of the 2D arrays was measured on a CSPM5500 scanning probe microscope in tapping mode (Benyuan, China). The optical images were acquired on a Leica TCS SP8 Confocal Microscope in bright field mode.Results and discussion
As outlined in Scheme 1, the 2D microgel CCs were fabricated by in situ fixing the first 111 plane of 3D microgel CCs via a benzophenone (BP)-based photochemical reaction. The PNIPAM microgels used in the study were prepared by free-radical precipitation polymerization.27 As reported previously, these microgels all experience a volume phase transition upon heating, as shown in Fig. S1.†25–27 Unless otherwise specified, the 1450 nm microgel was used in the following studies.Quartz slides were used as substrate to fabricate the 2D CCs. To introduce the photosensitizer, BP, onto the surface, the slides were first treated with a silane coupling agent with a carboxylic acid end functional group to introduce carboxylic acid groups. The carboxylic acid groups were then activated with EDC/NHS and coupled with 4-aminobenzophenone (ABP)41 (Scheme 2). The amount of carboxylic acid groups on quartz slide surface was determined by a colorimetric method based on Toluidine Blue O staining,41 which is 8.78 × 10−8 and 1.11 × 10−8 mol before and after ABP coupling (Fig. S2†). The result indicated that ∼87% carboxylic acid groups reacted with ABP.
Subsequently, 3D microgel CC was assembled on the functionalized quartz slides. For this purpose, the microgel dispersion was concentrated by centrifugation and then loaded in a cell composed of two quartz slides, one of which was functionalized with BP. As reported previously, the monodisperse microgel particles gradually self-assembled into highly ordered crystalline structure.46–49 As a result, the samples exhibit iridescent colors when illuminated with white light obliquely (Fig. 1A).50 The 3D assembly was examined with an optical microscope to investigate its crystal structure, as the microgel spheres are large enough to be visualized with an optical microscope. Fig. 1B shows a representative image, which indicates that the microgel spheres are arranged into a hexagonally close-packed crystalline array. The fast Fourier transformation (FFT) of the image shown in the inset confirms a structure with long-range order and hexagonal symmetry.51 In the image, the microgel spheres appear to be in intimate contact with one another. The average center-to-center distance between two adjacent particles was determined to be ∼1000 nm, which is smaller than the hydrodynamic diameter (Dh) of the microgel measured as free particles in solution. The different size of the microgel particles in the 3D assembly and solution suggests that the particles experienced a compression during centrifugation and crystallization.52
It is noteworthy that the image shown in Fig. 1B was taken from the interior part of the assembly. For the fabrication of 2D CC, the key is that the microgel particles close to the substrate should also self-assemble into ordered structure. Therefore the structure of the first layer of microgel particles close to substrate was studied. The image and the corresponding FFT shown in Fig. 1C reveals that these particles also form a highly ordered array, just like the particles in the interior region. Previously Lyon et al.52 also observed that microgel particles assemble into ordered structure along the container wall, which actually form the first 111 plane of the face-centered cubic (fcc) lattice of the 3D CC. The 111 planes in the internal region of the 3D crystal are all parallel to the substrate.51,52 Compared to the interior region, the degree of order close to the substrate is not reduced, suggesting the existence of the substrate have little influence on the assembly of the microgel particles. Previously Alsayed et al.53 even found that the container wall may stabilize the crystal and therefore the region close to the wall has less defects.
In order to fix the first layer of microgel particles onto the substrate, the crystallized sample was illuminated with UV light (Scheme 1). According to previous studies,39 when exposed to UV light, the BP chromophores on the substrate will absorb a photon at ∼350 nm, which will promote one electron from a nonbonding sp2-like n-orbital on oxygen to an antibonding π*-orbital of the carbonyl group (Scheme 3). The resulting triplet is biradical in nature which is ready to react with groups in its vicinity. Particularly the oxygen of the carbonyl group will abstract a hydrogen atom from the polymer chain of PNIPAM microgel particle, leaving behind two radicals, one at the previous carbonyl carbon and the other on the polymer chain. The two radicals will eventually recombine, leading to the covalent attachment of the microgel particles to the surface.
 | ||
| Scheme 3 Attachment of PNIPAM microgel particles onto quartz slide via photochemical reaction between BP chromophore and microgel particles. | ||
Fig. 2 shows the AFM and optical microscopy images of the resulting 2D arrays. Representative large area microscopy images were shown in Fig. S3 in the ESI.† As expected, arrays with highly ordered structure were obtained. The results suggest that the ordered structure of the microgel array close to the substrate was remained because the microgel particles were fixed onto the substrate in situ. In addition, because the particles were fixed via covalent bonds, the following washing in water and drying in the air do not disturb the pre-formed ordered structure.
To ensure that the successful fabrication of the ordered arrays is indeed due to the light-induced coupling of the surface BP groups to the polymer chains of the microgel particles, two control experiments were designed. In the first control, unmodified quartz slides were used instead of BP-modified ones. Similarly the samples were UV-irradiated followed by a washing procedure. As shown in Fig. 3A, some microgel particles were missing, suggesting they were washed away because of the relatively weak interaction between the particle and the substrate. In addition, the particles were not arranged in an ordered structure. In the second control, a BP-modified slide was used, but the UV irradiation step is omitted. Again some particles were found missing and the resulting array is unordered (Fig. 3B). These results indicate that BP modification of the substrate and UV irradiation are essential for the attachment of the microgel particles and maintaining the ordered structure. Previously the same photochemical reaction was exploited to attach various polymers onto BP-modified surfaces.40,41 It is noteworthy that the BP-based photochemical attachment was usually carried out in dry state.40,41 In the present case, however, the reaction undergoes in the presence of solvent (water). Despite of this, the images shown in Fig. 2 indicate that the microgel particles could still be effectively attached on the substrate. It was previously found that BPs react preferentially with unreactive C–H bonds, even in the presence of solvent water and bulk nucleophiles.39
 | ||
| Fig. 3 AFM images of 2D microgel arrays (A) using un-modified quartz slide as substrate, and (B) using BP-modified slide as substrate but without UV irradiation. Scan bar: 2 μm. | ||
In order to characterize the ordering of the 2D arrays quantitatively, the dimensionless pair correlation function, g(r), was calculated. g(r) is defined by the following equation:
 | (1) |
 | ||
| Fig. 4 (A) Pair correlation function, g(r), of the 2D CC from 1450 nm microgel. Dash vertical lines indicate the peaks of g(r) of an ideal hexagonally packed monolayer generated numerically. The inset highlights the centers of the particles as found from the automated particle location procedure. (B) Single-sided power spectra Fourier transforms (FT) of g(r) compared to FT of the corresponding perfectly ordered arrays. The power spectra were scaled to have identical maxima at f/f0 = 1.0.6 | ||
From g(r), another quantitative measure of ordering, i.e., the ratio of the full width at half maximum of the first peak in the Fourier transform of the function g(r) − 1, k, to that of a perfectly packed array, k0, can be extracted.6,54 As shown in Fig. 4B, the k/k0 value was determined to be 1.08. It is known that the ratio is 1 for an array with a perfectly packed structure, and it becomes larger with increasing disorder in the structure. Particularly a k/k0 less than 1.5 indicates a highly ordered structure, while a k/k0 larger than 1.5 indicates significant disordering.6,54 Here the k/k0 value is close to 1, suggesting the 2D microgel array is highly ordered.
The structure of the microgel arrays was also studied by laser diffraction, a convenient method to examine if the particles in a 2D array are arranged into ordered structure.50,55 If the particles form a perfect hexagonal crystal, when illuminated with monochromatic laser light, a diffraction pattern composed of a set of distinct spots with 6-fold symmetry will be obtained.55 As expected, the microgel CC displays a diffraction pattern with six symmetrical spots, confirming again its highly ordered structure (Fig. 5). Many previously reported 2D CCs display a diffraction pattern of a sharp circle (Debye ring), instead of distinct spots. The reason lies that the samples are composed of crystallites with a size significantly smaller than the beam diameter and the crystallites are randomly oriented.50,55 Here the 2D microgel CC displays a diffraction pattern with symmetrical distinct spots, suggesting that the microgel particles in the illuminated area (∼2 mm in diameter) are arranged perfectly into a hexagonal crystalline array.
Recently Bagheri et al. used a set of parameters to assess the ordering in colloidal crystals.56 Some of the parameters were also calculated for the 2D microgel CC (Table 2). Lindemann parameter, α, defined as the standard deviation of the distribution of displacements divided by the ideal lattice spacing, is a measure of the local positional disorder.57 This parameter was found to fall in a narrow range from 0.03 to 0.10 for CCs reported previously.56 For the 2D CC of the 1450 nm microgel, the parameter was calculated to be 0.042,58,59 falling in the same range for other CCs. Local bond orientational order for 6 fold symmetry, defined as
 | (2) |
| 2D microgel CCs | Image size (μm2) | α | Ψ6 | ξ6 |
|---|---|---|---|---|
| 880 nm microgel | 12 × 12 | 0.061 (±0.003) | 0.92 (±0.02) | 2.08 (±0.07) |
| 1450 nm microgel | 40 × 40 | 0.042 (±0.001) | 0.98 (±0.01) | 3.16 (±0.03) |
| 1550 nm microgel | 30 × 30 | 0.051 (±0.002) | 0.95 (±0.02) | 2.90 (±0.06) |
| 1800 nm microgel | 20 × 20 | 0.050 (±0.002) | 0.96 (±0.01) | 3.88 (±0.09) |
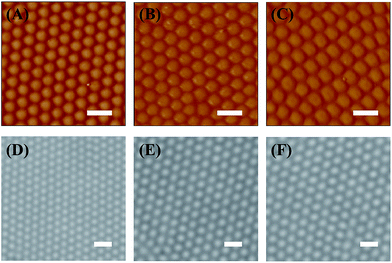
Besides the 1450 nm microgel, three other microgels, with size varying from 880 nm to 1800 nm, were also used to fabricate 2D arrays. The AFM and optical microscopy images of the resulting arrays were shown in Fig. 6. As the images indicated, all these microgels were arranged into a hexagonal crystalline array. Again the average center-to-center distance between two adjacent particles was found to be smaller than Dh of the microgel as free particles in solution. For example, the array shown in Fig. 6A and D was fabricated from microgel with a Dh of 880 nm, while the average interparticle distance of the array was ∼800 nm. The pair correlation function, g(r), of the 3 arrays was also calculated and shown in Fig. S4.† k/k0 value was determined to be 1.02, 1.38, and 1.48 for 2D array fabricated from 880 nm, 1550 nm, and 1800 nm microgel, respectively (Fig. S5†). These results confirm again a highly ordered structure of these arrays. Meanwhile, Lindemann parameter, α, local orientational bond order, Ψ6, and positional correlation length, ξ6, of the 2D microgel CCs were also calculated (Table 2). These values are comparable to the corresponding one of the CC from 1450 nm microgel.
To study the effect of the concentration of the dispersion, 2D arrays were fabricated using different concentration of 1450 nm microgel. As shown in Fig. 3, the interparticle distance of the 2D arrays decreases with increasing dispersion concentration. For the 2D array from 2.32 wt% dispersion, the interparticle distance is ∼1300 nm. This value reduces to be ∼1000 nm when the concentration increases to 3.62 wt%. From Scheme 1, the interparticle distance of the 2D array is determined by the interparticle distance of the 3D CC. It was previously revealed that the lattice constant of a 3D microgel CC can be tuned by varying the concentration of the microgel dispersion.46,47 In a higher concentration dispersion, the microgel particles will be compressed to a larger degree. Crystallization of the dispersion results in a 3D crystal with a reduced interparticle distance and hence a shorter lattice constant.46,47 Accordingly when the first 111 plane of the 3D CC is fixed in situ on the substrate, a 2D array with a shorter interparticle distance will be obtained.
It is highly desirable that a method can fabricate large scale 2D CCs. Unfortunately, most of the existing methods can only produce arrays with a relatively small size. Previously Asher et al.6 developed a method to assemble large area 2D arrays from hard colloids. An advantage of the method developed here is that it allows to produce 2D microgel CCs with any size, because 3D microgel CCs of any size can be facilely fabricated.37 In the above work we used quartz slides with a size of 4.5 cm × 2.5 cm as substrate. The area of the resulting 2D CC is ∼7.5 cm2. To demonstrate the ability to fabricate large size 2D CCs, a quartz slide with a size of 11 cm × 11 cm was used. The resulting 2D CC has an area of ∼61 cm2. When illuminated with white light obliquely from behind, the 2D CC displays vivid diffraction color (Fig. 7A), suggesting a highly ordered structure. Like other 2D CCs reported previously, different parts of the array display different colors, which can be explained by the different angle at which the individual part is illuminated.6,50 The polycrystalline nature of the array46 may be a second reason for the observed different colors.
 | ||
| Fig. 7 (A) Photograph of a 2D microgel CC fabricated using an 11 cm × 11 cm quartz slide as substrate. (B) A 2D microgel CC fabricated on the inner wall of a test tube. | ||
Most of the methods for 2D CC fabrication can only use planar substrates. In contrast, the method developed here allows for the fabrication of 2D CC on nonplanar substrates. As an example, the inner wall of a test tube with an inner diameter of 0.8 cm was modified with BP chromophore. Concentrated microgel dispersion was then added. After the dispersion crystallized, the tube was UV-irradiated. As shown in Fig. 7B, the tube also displays vivid diffraction color when illuminated with white light obliquely from behind. The result suggests that a crystalline array of microgel particles was successfully fabricated on the inner surface of the tube.
Conclusions
In conclusion, we fabricated 2D microgel CCs by first assembling the microgel spheres into a highly ordered 3D CC, followed by fixing the first 111 plane of the 3D CC onto the substrate via a BP-based photochemical reaction. In the 3D microgel CC, like ones in the interior region, the particles close to the substrate were also arranged into highly ordered structure. Under UV irradiation, covalent bonds forms between the BP groups on the substrate surface and the polymer chains of the microgel particles. As a result, the microgel particles close to the substrate were covalently bound onto the substrate. As they were fixed in situ, their highly ordered structure was maintained. Because large size 3D microgel CC can be facilely synthesized, this method allows for fabrication of large area 2D microgel CC. Beside planar substrate, 2D microgel CCs can also be fabricated on nonplanar substrates. In addition, the interparticle distance in the 2D CCs can be tuned by the concentration of the microgel dispersion. Because the BP-based photochemical reaction was chosen for the fixing purpose, instead of the photo-initiated thiol–ene reaction as we used before,38 the modification of the microgel particles is no longer needed.Acknowledgements
We thank financial support for this work from the National Natural Science Foundation of China (Grants No. 21274068 and 21374048), Tianjin Committee of Science and Technology (16JCZDJC32900), and PCSIRT program (IRT1257).Notes and references
- Y. N. Xia, B. Gates, Y. D. Yin and Y. Lu, Adv. Mater., 2000, 12, 693–713 CrossRef CAS
.
- H. Cong, B. Yu, J. Tang, Z. Li and X. Liu, Chem. Soc. Rev., 2013, 42, 7774–7800 RSC
.
- J. Zhang, Y. Li, X. Zhang and B. Yang, Adv. Mater., 2010, 22, 4249–4269 CrossRef CAS PubMed
.
- J. H. Holtz and S. A. Asher, Nature, 1997, 389, 829–832 CrossRef CAS PubMed
.
- S. Yang and Y. Lei, Nanoscale, 2011, 3, 2768–2782 RSC
.
- J. Zhang, L. Wang, D. N. Lamont, S. S. Velankar and S. A. Asher, Angew. Chem., Int. Ed., 2012, 51, 6117–6120 CrossRef CAS PubMed
.
- J. Zhang, L. Wang, J. Luo, A. Tikhonov, N. Kornienko and S. A. Asher, J. Am. Chem. Soc., 2011, 133, 9152–9155 CrossRef CAS PubMed
.
- D. Men, H. Zhang, L. Hang, D. Liu, X. Li, W. Cai, Q. Xiong and Y. Li, J. Mater. Chem. C, 2015, 3, 3659–3665 RSC
.
- F. Xue, Z. Meng, F. Wang, Q. Wang, M. Xue and Z. Xu, J. Mater. Chem. A, 2014, 2, 9559–9565 CAS
.
- N. Vogel, C. K. Weiss and K. Landfester, Soft Matter, 2012, 8, 4044–4061 RSC
.
- S. B. Quint and C. Pacholski, Soft Matter, 2011, 7, 3735–3738 RSC
.
- P. Jiang, T. Prasad, M. J. McFarland and V. L. Colvin, Appl. Phys. Lett., 2006, 89, 11908 CrossRef
.
- P. Jiang and M. J. McFarland, J. Am. Chem. Soc., 2004, 126, 13778–13786 CrossRef CAS PubMed
.
- P. Jiang and M. J. McFarland, J. Am. Chem. Soc., 2005, 127, 3710–3711 CrossRef CAS PubMed
.
- X. Ye and L. Qi, Sci. China: Chem., 2014, 57, 58–69 CrossRef CAS
.
- P. Born, A. Munoz, C. Cavelius and T. Kraus, Langmuir, 2012, 28, 8300–8308 CrossRef CAS PubMed
.
- M. Kargar, A. Pruden and W. A. Ducker, J. Mater. Chem. B, 2014, 2, 5962–5971 RSC
.
- R. Ye, Y. Ye, Z. Zhou and H. Xu, Langmuir, 2013, 29, 1796–1801 CrossRef CAS PubMed
.
- X. Sun, Y. Li, T. H. Zhang, Y. Ma and Z. Zhang, Langmuir, 2013, 29, 7216–7220 CrossRef CAS PubMed
.
- E. C. H. Ng, K. M. Chin and C. C. Wong, Langmuir, 2011, 27, 2244–2249 CrossRef CAS PubMed
.
- F. Xue, S. A. Asher, Z. Meng, F. Wang, W. Lu, M. Xue and F. Qi, RSC Adv., 2015, 5, 18939–18944 RSC
.
- F. Xue, Z. Meng, F. Qi, M. Xue and L. Qiu, Colloid Polym. Sci., 2016, 294, 479–482 CAS
.
- H. Yang, N. Gozubenli, Y. Fang and P. Jiang, Langmuir, 2013, 29, 7674–7681 CrossRef CAS PubMed
.
- Y. Xia, X. He, M. Cao, X. Wang, Y. Sun, H. He, H. Xu and J. R. Lu, Biomacromolecules, 2014, 15, 4021–4031 CrossRef CAS PubMed
.
- J. Y. A. K. Peter, Rep. Prog. Phys., 2014, 77, 56601 CrossRef PubMed
.
- Y. Guan and Y. Zhang, Soft Matter, 2011, 7, 6375–6384 RSC
.
- R. H. Pelton and P. Chibante, Colloids Surf., 1986, 20, 247–256 CrossRef CAS
.
- G. Zhang, D. Wang, Z. Gu, J. Hartmann and H. Möhwald, Chem. Mater., 2005, 17, 5268–5274 CrossRef CAS
.
- S. Schmidt, T. Hellweg and R. von Klitzing, Langmuir, 2008, 24, 12595–12602 CrossRef CAS PubMed
.
- S. Tsuji and H. Kawaguchi, Langmuir, 2005, 21, 8439–8442 CrossRef CAS PubMed
.
- Y. Lu and M. Drechsler, Langmuir, 2009, 25, 13100–13105 CrossRef CAS PubMed
.
- M. Horecha, V. Senkovskyy, A. Synytska, M. Stamm, A. I. Chervanyov and A. Kiriy, Soft Matter, 2010, 6, 5980–5992 RSC
.
- K. Horigome and D. Suzuki, Langmuir, 2012, 28, 12962–12970 CrossRef CAS PubMed
.
- K. Geisel, W. Richtering and L. Isa, Soft Matter, 2014, 10, 7968–7976 RSC
.
- N. Vogel, C. Fernandez-Lopez, J. Perez-Juste, L. M. Liz-Marzan, K. Landfester and C. K. Weiss, Langmuir, 2012, 28, 8985–8993 CrossRef CAS PubMed
.
- A. S. Iyer and L. A. Lyon, Angew. Chem., Int. Ed., 2009, 48, 4562–4566 CrossRef CAS PubMed
.
- T. Hellweg, Angew. Chem., Int. Ed., 2009, 48, 6777–6778 CrossRef CAS PubMed
.
- X. Li, J. Weng, Y. Guan and Y. Zhang, Langmuir, 2016, 32, 3977–3982 CrossRef CAS PubMed
.
- G. Dorman and G. D. Prestwich, Biochemistry, 1994, 33, 5661–5673 CrossRef CAS PubMed
.
- O. Prucker, C. A. Naumann, J. Rühe, W. Knoll and C. W. Frank, J. Am. Chem. Soc., 1999, 121, 8766–8770 CrossRef CAS
.
- N. Singh, A. W. Bridges, A. J. Garcia and L. A. Lyon, Biomacromolecules, 2007, 8, 3271–3275 CrossRef CAS PubMed
.
- M. D. Barker, R. A. Dixon, S. Jones and B. J. Marsh, Tetrahedron, 2006, 62, 11663–11669 CrossRef CAS
.
- B. E. Campbell, M. Tarleton, C. P. Gordon, J. A. Sakoff, J. Gilbert, A. McCluskey and R. B. Gasser, Bioorg. Med. Chem. Lett., 2011, 21, 3277–3281 CrossRef CAS PubMed
.
- S. Sam, L. Touahir, J. Salvador Andresa, P. Allongue, J. N. Chazalviel, A. C. Gouget-Laemmel, C. Henry De Villeneuve, A. Moraillon, F. Ozanam, N. Gabouze and S. Djebbar, Langmuir, 2010, 26, 809–814 CrossRef CAS PubMed
.
- B. Gupta, C. Plummer, I. Bisson, P. Frey and J. Hilborn, Biomaterials, 2002, 23, 863–871 CrossRef CAS PubMed
.
- J. D. Debord, S. Eustis, S. B. Debord, M. T. Lofye and L. A. Lyon, Adv. Mater., 2002, 14, 658–662 CrossRef CAS
.
- J. Gao and Z. B. Hu, Langmuir, 2002, 18, 1360–1367 CrossRef CAS
.
- M. Chen, Y. Zhang, S. Jia, L. Zhou, Y. Guan and Y. Zhang, Angew. Chem., Int. Ed., 2015, 54, 9257–9261 CrossRef CAS PubMed
.
- M. Chen, L. Zhou, Y. Guan and Y. Zhang, Angew. Chem., Int. Ed., 2013, 52, 9961–9965 CrossRef CAS PubMed
.
- B. G. Prevo and O. D. Velev, Langmuir, 2004, 20, 2099–2107 CrossRef CAS PubMed
.
- J. Brijitta, B. V. R. Tata, R. G. Joshi and T. Kaliyappan, J. Chem. Phys., 2009, 131, 74904–74908 CrossRef CAS PubMed
.
- S. B. Debord and L. A. Lyon, J. Phys. Chem. B, 2003, 107, 2927–2932 CrossRef CAS
.
- A. M. Alsayed, M. F. Islam, J. Zhang, P. J. Collings and A. G. Yodh, Science, 2005, 309, 1207–1210 CrossRef CAS PubMed
.
- R. Rengarajan, D. Mittleman, C. Rich and V. Colvin, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2005, 71, 16615 CrossRef PubMed
.
- J. Zhang, X. Chao, X. Liu and S. A. Asher, Chem. Commun., 2013, 49, 6337–6339 RSC
.
- P. Bagheri, A. M. Almudallal, A. Yethiraj and K. M. Poduska, Langmuir, 2015, 31, 8251–8259 CrossRef CAS PubMed
.
- T. Speck, H. Löwen and J. Bialké, Phys. Rev. Lett., 2012, 108, 168301 CrossRef PubMed
.
- Y. Zhou, M. Karplus, K. D. Ball and R. S. Berry, J. Chem. Phys., 2002, 116, 2323–2329 CrossRef CAS
.
- Z. Wang, A. M. Alsayed, A. G. Yodh and Y. Han, J. Chem. Phys., 2010, 132, 154501 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Additional characterization of microgel and microgel CCs. See DOI: 10.1039/c6ra18622j |
| This journal is © The Royal Society of Chemistry 2016 |