Dendrimer-functionalized LAPONITE® nanodisks loaded with gadolinium for T1-weighted MR imaging applications†
Rania Mustafa‡
a,
Benqing Zhou‡a,
Jia Yang‡b,
Linfeng Zhengb,
Guixiang Zhang*b and
Xiangyang Shi *ac
*ac
aState Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Chemistry, Chemical Engineering and Biotechnology, Donghua University, Shanghai 201620, People's Republic of China. E-mail: xshi@dhu.edu.cn
bDepartment of Radiology, Shanghai General Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai 200080, People's Republic of China. E-mail: guixiangzhang@sina.com
cCQM-Centro de Química da Madeira, Universidade da Madeira, Campus da Penteada, 9000-390 Funchal, Portugal
First published on 30th September 2016
Abstract
In this report, we present a facile approach to forming dendrimer-functionalized LAPONITE® (LAP) nanodisks loaded with gadolinium (Gd) for in vitro and in vivo T1-weighted MR imaging applications. In this work, LAP nanodisks were sequentially modified with silane coupling agents, succinic anhydride to have abundant carboxyl groups on their surface, and amine-terminated poly(amidoamine) (PAMAM) dendrimers of generation 2 (G2). The dendrimer-modified LAP nanodisks were then conjugated with gadolinium (Gd) chelator diethylenetriaminepentaacetic acid (DTPA), followed by Gd(III) chelation to form the LM–G2–DTPA (Gd) nanocomplexes. The designed LM–G2–DTPA(Gd) nanocomplexes were characterized via different techniques. Cell viability assay shows that the formed LM–G2–DTPA(Gd) nanodisks are non-cytotoxic in the given concentration range. With a high r1 relaxivity (2.05 mM−1 s−1), the LM–G2–DTPA(Gd) nanocomplexes are able to be used as an efficient contrast agent for T1-weighted MR imaging of cancer cells in vitro and animal organ/tumor model in vivo. The designed LM–G2–DTPA(Gd) nanocomplexes may hold a great promise to be used as a versatile nanoplatform for MR imaging of different biological systems.
Introduction
Molecular imaging (MI) has become an indispensable tool for disease diagnosis, and can provide physiological and pathological information of the disease site. Various imaging modalities have been developed including magnetic resonance (MR) imaging,1–3 computed tomography (CT),4,5 positron emission tomography,6,7 and fluorescence imaging8,9 etc. Among them, MR imaging is a powerful noninvasive technique with high spatial resolution and tomography capabilities, which provides wonderful soft tissue contrast.10 For high-quality MR imaging, contrast agents have to be used. Recent advances in nanotechnology have shown that various nanoparticles (NPs) have been widely used in disease diagnosis,11–13 especially for MR imaging.14,15Based on different models of relaxation, MR imaging contrast agents could be divided into longitudinal (T1)-positive agents and transverse (T2)-negative agents, which give brighter images in T1-weighted MR imaging and darker images in T2-weighted MR imaging, respectively when compared with images without the use of contrast agents. Classically, gadolinium (Gd)- or manganese (Mn)-based T1 contrast agents, and superparamagnetic iron oxide NP-based T2 contrast agents have been well developed.16–18 T1-weighted contrast agents have predominant positive signal-enhancing ability to generate high spatial resolution.3,19,20 Currently, the clinically used MR imaging contrast agents mainly are Gd(III)-based complexes, such as Magnevist (Gd–DTPA) and Dotarem (Gd–DTOA).21 Unfortunately, these Gd(III)-based small molecular contrast agents are normally cleared rapidly by kidney and liver, resulting in short imaging time. Therefore, the contrast enhancement time window for MR imaging is not sufficiently wide, limiting the acquisition of the high resolution images of angiography as well as organs. In order to overcome the drawbacks of these small molecular contrast agents, a large number of Gd(III)-loaded macromolecular contrast agents have been introduced to increase their blood circulation time and resolution.15,21,22 Although these formed contrast agents have been demonstrated to have prolonged circulation time and enhanced MR contrast effect with the dramatically increased Gd(III) loading, their translation to clinical applications has often been hindered due to the complicated synthesis and the use of expensive carrier systems. Therefore, development of a facile strategy to synthesize Gd(III)-loaded macromolecular MR imaging contrast agents with the translational medicine potential still remains a great challenge.
LAPONITE® (LAP) nanodisks, with a dimension of 25 nm in diameter and 1 nm in thickness, are a type of cost-effective isomorphous synthetic clay material with a similar composition and structure to the natural clay mineral of hectorite.23–26 Recently, it has been demonstrated that LAP nanodisks have been widely used as carriers for drug delivery and MI.27–29 For example, LAP nanodisks can be applied to incorporate with iron oxide NPs for T2-weighted MR imaging of tumors,30 and can be linked with a conventional iodinated CT contrast agent diatrizoic acid for CT imaging of organs and tumors.31 LAP nanodisks27 or LAP nanodisks modified with silane,32 polyethylene glycol,33 or generation 2 (G2) poly(amidoamine) dendrimers34 can be used as a drug carrier to load anticancer drug doxorubicin for cancer chemotherapy. These prior successes regarding the use of LAP nanodisks as an effective nanocarrier to generate functional nanocomplexes for drug delivery and MI lead us to speculate that LAP nanodisks may be combined with diethylenetriaminepentaacetic acid (DTPA) for Gd(III) chelation, providing a unique contrast agent for MR imaging applications.
In the present study, LAP nanodisks were first silanized, carboxylated to have abundant carboxyl groups, and modified with G2 dendrimers via 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride acid (EDC) chemistry. The dendrimer-modified LAP nanodisks were then functionalized with DTPA ligands, followed by Gd(III) chelation to form the LM–G2–DTPA(Gd) nanocomplexes (Scheme 1). The nanocomplexes were characterized via different techniques. Their cytocompatibility was evaluated by quantitative cell viability assay. The r1 relaxivity measurement was used to assess the possibility to use the complexes for MR imaging. Finally, the LM–G2–DTPA(Gd) nanocomplexes were used for MR imaging of cancer cells in vitro and major organs and a tumor model in vivo. To the best of our knowledge, this is the first report regarding the use of dendrimer-modified LAP nanodisks for MR imaging applications.
Experimental
Materials
LAP nanodisks were purchased from Zhejiang Institute of Geology and Mineral Resources (Hangzhou, China). Ethylenediamine core G2 PAMAM dendrimers (Mw = 3256) were supplied from Dendritech (Midland, MI). EDC and N-hydroxysuccinimide (NHS) were purchased from J&K Chemical Ltd. (Shanghai, China). Triethylamine, dimethyl sulfoxide (DMSO), amino-propyldimethylethoxysilane (APMES), Cell Counting Kit-8 (CCK-8), and all the other chemicals and solvents were purchased from Aldrich (St. Louis, MO). HeLa cells (a human cervical carcinoma cell line) were obtained from Institute of Biochemistry and Cell Biology (the Chinese Academy of Sciences, Shanghai, China). Dulbecco's modified eagle medium (DMEM) was from Hangzhou Jinuo Biomedical Technology (Hangzhou, China). Fetal bovine serum (FBS), penicillin, and streptomycin were purchased from HyClone Lab., Inc. (Logan, UT). All chemicals and materials were used as received. Water used in all experiments was purified using a Milli Q Plus 185 water purification system (Millipore, Bedford, MA) with a resistivity higher than 18.2 MΩ cm. Regenerated cellulose dialysis membranes with a molecular weight cut-off (MWCO) of 1000 or 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 were acquired from Fisher (Pittsburgh, PA).
000 were acquired from Fisher (Pittsburgh, PA).
Synthesis of LM–G2 nanodisks
According to a similar approach that reported in our previous work27,34 and also as shown in Scheme 1, LAP powder (50 mg) was dispersed in 40 mL water at 50 °C overnight under magnetic stirring to form an LAP aqueous solution (1.25 mg mL−1). APMES (2%, 2 mL) aqueous solution was added dropwise to 5 mL of LAP solution under vigorous stirring at 50 °C for 16 h. Then the mixture was dialyzed against water (nine times, 2 L) for three days using a dialysis membrane with an MWCO of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The obtained LM–NH2 aqueous solution was stored at 4 °C before use. The above LM–NH2 solution (5 mL in DMSO) was added with succinic anhydride (SAH) (13.7 mg, in 5 mL DMSO) under vigorous stirring for 24 h and purified to form the LM–COOH. The formed LM–COOH solution (5 mg, in 4 mL PBS) was mixed with EDC (5 mg, in 2 mL DMSO) and NHS (3 mg, in 2 mL DMSO) for 3 h to active the carboxyl groups. Then, the activated LM–COOH solution was added dropwise into a G2 solution (3.7 mg mL−1, in 5 mL water) and stirred for 3 days at room temperature to form the LM–G2. The reaction mixture was dialyzed against PBS (three times, 2 L) and water (three times, 2 L) using a dialysis bag with an MWCO of 14
000. The obtained LM–NH2 aqueous solution was stored at 4 °C before use. The above LM–NH2 solution (5 mL in DMSO) was added with succinic anhydride (SAH) (13.7 mg, in 5 mL DMSO) under vigorous stirring for 24 h and purified to form the LM–COOH. The formed LM–COOH solution (5 mg, in 4 mL PBS) was mixed with EDC (5 mg, in 2 mL DMSO) and NHS (3 mg, in 2 mL DMSO) for 3 h to active the carboxyl groups. Then, the activated LM–COOH solution was added dropwise into a G2 solution (3.7 mg mL−1, in 5 mL water) and stirred for 3 days at room temperature to form the LM–G2. The reaction mixture was dialyzed against PBS (three times, 2 L) and water (three times, 2 L) using a dialysis bag with an MWCO of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for three days, followed by lyophilization to get the product of LM–G2.
000 for three days, followed by lyophilization to get the product of LM–G2.
Formation of LM–G2–DTPA(Gd) complexes
DTPA (18.1 mg, 4 mL DMSO) was added to the LM–G2 solution (15 mg, 4 mL in water) under vigorous stirring for 3 days at room temperature. The formed LM–G2–DTPA was purified using dialysis membrane with an MWCO of 1000 for three days. Then, Gd(NO3)3·6H2O (45.1 mg, 5 mL water) with 11 molar equiv. of the G2 dendrimer was added into the LM–G2–DTPA solution under vigorous stirring for 24 h. The excess Gd(III) was removed by dialysis against water (nine times, 2 L) for three days with a membrane having an MWCO of 1000, followed by lyophilization to obtain the LM–G2–DTPA(Gd) nanocomplexes.Characterization techniques
Thermogravimetric analysis (TGA) was carried out using a TG209F1 thermo-gravimetric analyzer (Netzsch, Germany). Samples were heated from room temperature to 700 °C at a rate of 20 °C min−1 under a nitrogen atmosphere. Zeta potential and dynamic light scattering (DLS) measurements were carried out using a Malvern Zetasizer Nano ZS model ZEN3600 (Worcestershire, UK) equipped with a standard 633 nm laser. Fourier transform infrared (FTIR) spectroscopy was performed using a Nicolet Nexus 670 FTIR spectrophotometer (Nicolet-Thermo, Waltham, MA). All spectra were recorded using a transmission mode with a wavenumber range of 650–4000 cm−1. Transmission electron microscopy (TEM) was performed using a JEOL 2010F analytical electron microscope (JEOL, Tokyo, Japan) operating at 200 kV. Transmission electron microscopy (TEM) samples were prepared by depositing a particle suspension (0.5 mg mL−1, 5 μL) onto a carbon-coated copper grid and air-dried before the measurements. The size and morphology of the LAP–G2–DTPA(Gd) nanocomplexes were also observed by atomic force microscopy (AFM, Veeco DI-NanoScopy IV, Santa Barbara, CA) using tapping mode and a rotated monolithic silicon probe. The parameters were set as following: Imaging Mode (AC mode), scan lines (256), scan points (256), scan rate (1.00 Hz), and scan size (3.00 μm). The Gd content was determined by a Leeman Prodigy inductively coupled plasma-optical emission spectroscopy (ICP-OES, Hudson, NH). T1 relaxometry of the LM–G2–DTPA(Gd) nanocomplexes was performed using a 3.0 T Signa HDxt superconductor MR system (GE Medical Systems, Milwaukee, WI) with a wrist receiver coil. T1 relaxation times were measured using an SE/2D sequence. A total of four echoes were used with the following parameters: TR = 300, 600, 900, 1200 ms, TE = 10.7 ms, matrix = 256 × 256, section thickness = 2 mm, and FOV = 12 cm. The T1 relaxivity (r1) was determined by linear fitting of the inverse relaxation times as a function of the Gd(III) concentration.Cell culture and cytotoxicity assay
HeLa cells were regularly cultured in a 25 mL culture flask with 5 mL of DMEM containing 10% FBS, 100 U mL−1 penicillin, and 100 μg mL−1 streptomycin in a humidified incubator with 5% CO2 at 37 °C. For cytotoxicity assay, HeLa cells with a density of 1 × 104 cells per well were seeded into each well of a 96-well plate and allowed to grow overnight to bring the cells to confluence. Then the medium was replaced with fresh medium containing PBS or LM–G2–DTPA(Gd) with a final Gd concentration ranging from 12.5 to 200 μg mL−1. After 24 h incubation, the cells were washed with PBS for 3 times, and then 100 μL fresh medium containing CCK-8 (10 μL, 7sea biotech. Co., Ltd., Shanghai, China) was added to each well. After incubation at 37 °C for 4 h, the plates were read at 450 nm using an MK3 Microplate Reader (PerkinElmer, Waltham, MA). Mean and standard deviation for the triplicate wells for each sample were reported.In vitro T1-weighted MR imaging of cancer cells
Approximately 4 × 107 HeLa cells were plated into each well of a 6-well plate with 2 mL of DMEM at 37 °C and 5% CO2 for 12 h to bring the cells to confluence. Then the medium was replaced with 3 mL fresh medium containing PBS (as control) or LM–G2–DTPA(Gd) at different Gd concentrations (2.2, 1.1, 0.55, 0.27, and 0.14 mM, respectively). After incubation at 37 °C and 5% CO2 for 6 h, the cells were then trypsinized, centrifuged, resuspended in 100 μL PBS, and placed in 2 mL Eppendorf tubes before MR imaging. The MR imaging parameters are similar to those used in animal imaging (see below).In vivo MR imaging
All animal experiments were carried out following the protocols approved by the Ethical Committee of Shanghai General Hospital and the policy of the National Ministry of Health. For MR imaging, Kunming mice (15–20 g, Shanghai Slac Laboratory Animal Center, Shanghai, China) were anesthetized by intraperitoneal injection of pentobarbital sodium (40 mg kg−1). An aqueous solution of LM–G2–DTPA(Gd) ([Gd] = 2.2 mM, in 0.1 mL PBS solution) was delivered to a mouse via the tail vein. A 3.0 T Signa HDxt superconductor clinical MR system was used with a custom-built rodent receiver coil (Chenguang Med Tech, Shanghai, China). The mouse to be imaged was placed inside the MR receiver coil. At each time point for each animal, two-dimensional (2D) spin-echo MR images were obtained with 2 mm slice thickness, TR/TE 2000/81.9 ms, 6 × 6 cm FOV, and 256 × 160 matrix. 2D MR images were obtained both before and after administration of the LM–G2–DTPA(Gd) nanocomplexes at the time points of 15, 30, 45, and 60 min, respectively. The total time to acquire the 2D images was 2.2 min.In vivo MR imaging of HeLa tumor model
To establish the xenografted tumor model, 1.5 × 106 HeLa cells were subcutaneously injected into the right hind leg of BALB/c nude mice (15–20 g), which were purchased from Shanghai Slac Laboratory Animal Center, Shanghai, China. When the tumor volume reached about 0.8–1.0 cm3 at three weeks postinjection, the mice were anesthetized by intraperitoneal injection of pentobarbital sodium (40 mg kg−1), and then the LM–G2–DTPA(Gd) nanocomplexes ([Gd] = 2.2 mM, in 0.15 mL PBS) were injected into the mice via the tail vein. MR images were obtained pre- and post-injection at the time points of 15, 30, 45, and 60 min using a 3.0 T Signa HDxt superconductor clinical MR with the above mentioned parameters.Results and discussion
Synthesis and characterization of the LM–G2–DTPA(Gd) nanocomplexes
Our previous work has shown that LM–G2 nanodisks can be used as a versatile drug delivery platform.34 Logically, in this present study we attempted to use the LM–G2 nanodisks loaded with Gd(III) for MR imaging applications (Scheme 1). In this work, LAP nanodisks were modified with silane coupling agents and succinic anhydride to have abundant carboxyl groups on their surface. Then, G2 PAMAM dendrimers were modified onto the surface of LAP nanodisks. The dendrimer-modified LAP nanodisks were linked with DTPA, followed by Gd(III) chelation to form the LM–G2–DTPA(Gd) nanocomplexes. The designed LM–G2–DTPA(Gd) nanocomplexes were characterized via different techniques.As shown in Fig. 1, compared with pristine LAP, the weight loss of LM–NH2 in the temperature ranging from 200 to 600 °C was 8.3%, indicating that 8.3% APMES has been modified on the surface of LAP. In addition, LM–G2 has about 34.7% weight loss by subtracting the remnant weight of LM–NH2 at 600 °C, which may be attributed to the amount of G2 dendrimers modified onto the LAP nanodisks. Furthermore, by subtracting the remnant weight of LM–G2 at 600 °C, LM–G2–DTPA shows about 5.3% weight loss, which is probably ascribed to the amount of DTPA modified onto the dendrimers. Therefore, the TGA results indicate that LM–NH2, LM–G2, and LM–G2–DTPA have been successfully synthesized.
The surface potential and hydrodynamic size of these samples were measured by zeta potential and DLS (Fig. 2). It is clear that LAP has a negative surface potential of −33.5 ± 0.6 mV, which is increased to −18.0 ± 0.8 mV after silanization to form the LM–NH2. After the subsequent reaction with SAH, the zeta potential of the LM–COOH decreases to −20.6 ± 0.4 mV, which was reversed backed to a positive surface potential of 13.4 ± 0.2 mV after conjugation with G2 dendrimer (LM–G2). This indicates the successful modification of G2 dendrimer onto the surface of the LAP nanodisks. Further modification with DTPA onto the surface of LM–G2 results in a surface potential of 11.0 ± 0.2 mV, which can be explained by the partial surface coverage of the particles with the negatively charged DTPA. Moreover, after Gd(III) complexation, the LM–G2–DTPA(Gd) nanocomplexes display a surface potential up to 18.0 ± 0.2 mV. The changes of surface potential of the LAP nanodisks after each step of surface modification demonstrate the step-by-step modification as designed. It should be noted that due to the fact that the surface potential of the nanocomplexes is just slightly positive, significant cytotoxicity of the material is not expected, which can be confirmed by cell viability assay (see below).
 | ||
| Fig. 2 Zeta-potential (a) and hydrodynamic size (b) of the LAP (1), LM–NH2 (2), LM–COOH (3), LM–G2 (4), LM–G2–DTPA (5), and LM–G2–DTPA(Gd) (6), respectively. | ||
Meanwhile, the hydrodynamic diameter of the LAP nanodisks increases after each step of modification (Fig. 2b). The silanization reaction enabled a size increase from 74.0 ± 1.0 nm (LAP) to 203.0 ± 0.5 nm (LM–NH2), suggesting a certain degree of aggregation of the nanodisks. Further conjugation of G2 dendrimers led to an increased size of 750.0 ± 1.0 nm for the nanodisks. This is likely because G2 dendrimers not only modify the surface of a single LAP nanodisks, but also act as a bridge to promote the formation of clustered LAP structure in aqueous solution. After grafting with DTPA and further chelation of Gd(III), the size of the LAP nanodisks was increased to 850.0 ± 2.0 nm and 905.0 ± 2.0 nm, respectively. It is noted that the measured large hydrodynamic size of the LM–G2–DTPA(Gd) nanocomplexes (900 nm) is inconsistent with that of the pristine LAP nanodisks (25 nm). This can be ascribed to the fact that the mean diameter of LAP (25 nm) is measured by TEM, and TEM is generally used to measure the size of a single particle in a dry state. In contrast, the hydrodynamic size of the LM–G2–DTPA(Gd) nanocomplexes was measured by DLS, and DLS reflects the size of particle clusters or aggregated particles dispersed in aqueous solution that may consist of many single particles.31 The LM–G2–DTPA(Gd) nanocomplexes with a large hydrodynamic size does not impact their cellular uptake behavior, in agreement with our previous work.27,34
The formation of the LM–G2–DTPA(Gd) nanocomplexes was also qualitatively verified by FTIR spectrometry (Fig. 3). In the spectra of LAP and LM–NH2, the band at 1260 cm−1 could be attributed to the irregular stretching vibration of the Si–O bond, indicating the successful silanization reaction.32,34,35 The strong peak at 3389 cm−1 can be assigned to the –OH groups of LAP. After the conjugation with G2, the strong peak at 3207 cm−1 can be attributed to the N–H stretching vibration of amide groups of G2, and 2916 cm−1 can be assigned to the –CH2– stretching vibration of methylene group of G2 dendrimers.34,36,37 After the modification of DTPA, a strong peak at 1623 cm−1 can be assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch vibrations of DTPA. In the spectra of the LM–G2–DTPA(Gd), the peaks at the 1626 cm−1 and 1595 cm−1 are attributed to asymmetric and symmetric stretching modes of the carboxyl groups of DTPA, respectively.17 Furthermore, the morphology of the LAP nanodisks after each step of surface modification was observed via TEM (Fig. 4). Clearly, the increased tendency of aggregation after each further surface modification step can be observed, in agreement with the DLS data. Furthermore, the size and morphology of the formed LM–G2–DTPA(Gd) nanocomplexes were also observed by AFM (Fig. S1, ESI†). Taken together with the TEM data, the disk shape of the LM–G2–DTPA(Gd) nanocomplexes can be confirmed. In addition, the LM–G2–DTPA(Gd) nanocomplexes display good colloidal stability when dispersed in water, PBS, and cell culture medium, and no precipitation occurred for at least seven days when stored at 4 °C (Fig. S2, ESI†).
O stretch vibrations of DTPA. In the spectra of the LM–G2–DTPA(Gd), the peaks at the 1626 cm−1 and 1595 cm−1 are attributed to asymmetric and symmetric stretching modes of the carboxyl groups of DTPA, respectively.17 Furthermore, the morphology of the LAP nanodisks after each step of surface modification was observed via TEM (Fig. 4). Clearly, the increased tendency of aggregation after each further surface modification step can be observed, in agreement with the DLS data. Furthermore, the size and morphology of the formed LM–G2–DTPA(Gd) nanocomplexes were also observed by AFM (Fig. S1, ESI†). Taken together with the TEM data, the disk shape of the LM–G2–DTPA(Gd) nanocomplexes can be confirmed. In addition, the LM–G2–DTPA(Gd) nanocomplexes display good colloidal stability when dispersed in water, PBS, and cell culture medium, and no precipitation occurred for at least seven days when stored at 4 °C (Fig. S2, ESI†).
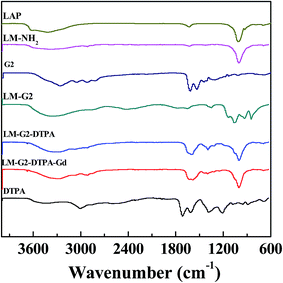 | ||
| Fig. 3 FTIR spectra of LAP, LM–NH2, G2, LM–G2, LM–G2–DTPA, DTPA, and LM–G2–DTPA(Gd) NPs, respectively. | ||
 | ||
| Fig. 4 TEM images of LAP (a), LM–NH2 (b), LM–G2 (c), LM–G2–DTPA (d), and LM–G2–DTPA(Gd) (e), respectively. | ||
Cytotoxicity assay of the LM–G2–DTPA(Gd) nanocomplexes
We next assessed the cytotoxicity of the LM–G2–DTPA(Gd) nanocomplexes via CCK-8 assay of HeLa cells (Fig. 5). It is clear that cells treated with the LM–G2–DTPA(Gd) in a concentration range of 0–200 μg mL−1 have approximately similar viability to the PBS control (p > 0.05, n = 3). This suggests that the designed LM–G2–DTPA(Gd) nanocomplexes do not display apparent cytotoxicity in the studied concentration range. | ||
| Fig. 5 CCK-8 assay of HeLa cells treated with LM–G2–DTPA(Gd) at different concentrations (12.5–200 μg mL−1) for 24 h at 37 °C. | ||
T1-Weighted MR relaxometry
The presence of Gd(III) ions endowed the LM–G2–DTPA(Gd) nanocomplexes with r1 relaxivity for MR imaging applications. MR phantom studies show that the MR signal intensity of the LM–G2–DTPA(Gd) increases with the Gd concentration in the MR images (Fig. 6a). By linear fitting the inverse T1 as a function of Gd concentration (Fig. 6b), we were able to calculate the r1 relaxivity of the LM–G2–DTPA(Gd) to be 2.05 mM−1 s−1, which is about 1.5 times higher than that of DTPA(Gd) complexes (1.4 mM−1 s−1).38 | ||
| Fig. 6 T1-Weighted MR images (a) and linear fitting (b) of LM–G2–DTPA(Gd) at different Gd concentrations. | ||
MR imaging of cancer cells in vitro
With the high r1 relaxivity, the designed LM–G2–DTPA(Gd) nanocomplexes were firstly used to detect cancer cells via T1-weighted MR imaging (Fig. 7). It is clear that the MR signal intensity of HeLa cells incubated with the LM–G2–DTPA(Gd) nanocomplexes becomes brighter with the increase of the Gd concentration (Fig. 7a). Quantitative analysis of the MR signal intensity of the cell MR images (Fig. 7b) reveals that the MR signal intensity of the cells treated with the LM–G2–DTPA(Gd) complexes increases with the Gd concentration. These results suggest that the cells are able to uptake the LM–G2–DTPA(Gd) complexes likely via two distinct mechanisms (phagocytosis or diffusion via cell walls),39,40 allowing for effective MR imaging of cancer cells in vitro. | ||
| Fig. 7 T1-Weighted MR images (a) and MR signal intensity (b) of HeLa cells incubated with LM–G2–DTPA(Gd) at different Gd concentrations for 6 h. | ||
MR imaging of animal organs
We further assessed the potential to use the LM–G2–DTPA(Gd) nanocomplexes for T1-weighted MR imaging of animal organs (Fig. 8). Clearly, the liver and bladder of the mouse become brightened after injection of the LM–G2–DTPA(Gd) complexes, suggesting that the LM–G2–DTPA(Gd) complexes can be used for T1-weighted MR imaging of liver and bladder. The MR contrast enhancement can be maintained for at least 60 min postinjection.Quantitative analysis of the MR signal intensity (Fig. 8b) shows that the MR signal intensity of the liver firstly increases from 273 ± 3.6 (before injection) to 573 ± 3.8 (45 min postinjection), and then begins to decrease to 448 ± 4.1 at 60 min postinjection, while the MR signal intensity of the bladder increases gradually with time postinjection. At 60 min postinjection, the MR signal intensity of the bladder is up to 811 ± 4.2, which is significantly higher than that of the bladder before injection (303 ± 4.4). These findings validate that the formed LM–G2–DTPA(Gd) nanocomplexes can be used as a contrast agent for effective MR imaging of animal organs.
MR imaging of tumors
We further investigated the feasibility to use the LM–G2–DTPA(Gd) nanocomplexes for in vivo MR imaging of an animal tumor model (Fig. 9). Clearly, the MR images acquired before administration of the complexes have little inherent contrast between the tumor tissue and the surrounding muscle (Fig. 9a). At 15 min post administration, the subcutaneous tumor appears brighter than the adjacent tissues. After that, the tumor lesion steadily becomes clearer and sharper with the time postinjection and the image shows accurate delineation of the tumor boundary at 60 min postinjection. This suggests that the LM–G2–DTPA(Gd) complexes are able to efficiently penetrate in the tumor site likely through the enhanced permeation and retention (EPR) effect.5,17,40 Quantitatively, the signal intensity of the tumor region increases gradually and finally reaches 475 ± 3.8 at 60 min postinjection (Fig. 9b). This excellent tumor MR imaging performance may be due to the relatively long circulating time as well as the good r1 relaxivity of the nanocomplexes. It should be noted that although the developed nanocomplexes have a large hydrodynamic size, they could perform well as single particles that can be uptaken by cells and be accumulated into the tumor region, allowing for effective MR imaging of cancer cells in vitro and tumor model in vivo.Conclusion
In summary, a facile strategy has been developed to construct LM–G2–DTPA(Gd) nanocomplexes for in vitro and in vivo MR imaging applications. Through the dendrimer modification, Gd(III) ions can be effectively loaded onto the surface of LAP nanodisks, and the formed LM–G2–DTPA(Gd) nanocomplexes exhibit a higher r1 relaxivity (2.05 mM−1 s−1) than DTPA(Gd) complexes. The developed LM–G2–DTPA(Gd) nanocomplexes can be used as a contrast agent for efficient MR imaging of cancer cells in vitro and animal organs and tumor model in vivo. With the proven good cytocompatibility, the developed LM–G2–DTPA(Gd) nanocomplexes may be used as a promising contrast agent for T1-weighted MR imaging of various biological systems. Moreover, we also expect that multifunctional LAP nanodisks may be developed as a versatile platform for targeted imaging and drug or gene delivery for tumor theranostics due to the high loading capacity of the LAP.Acknowledgements
This research is financially supported by the National Natural Science Foundation of China (21273032, 81501518, 81271384, and 81371623) and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning. B. Z. thanks the support from the Chinese Universities Scientific Fund (CUSF-DH-D-2014034). X. Shi acknowledges the support of the Portuguese Science Foundation (FCT) through the CQM pluriannual base funding Project PEst-UID/QUI/00674/2013.Notes and references
- J. Gao, G. Liang, J. S. Cheung, Y. Pan, Y. Kuang, F. Zhao, B. Zhang, X. Zhang, E. X. Wu and B. Xu, J. Am. Chem. Soc., 2008, 130, 11828–11833 CrossRef CAS PubMed.
- L. S. Karfeld-Sulzer, E. A. Waters, N. E. Davis, T. J. Meade and A. E. Barron, Biomacromolecules, 2010, 11, 1429–1436 CrossRef CAS PubMed.
- M. Marradi, D. Alcantara, J. Martinez de la Fuente, M. Luisa Garcia-Martin, S. Cerdan and S. Penades, Chem. Commun., 2009, 3922–3924 RSC.
- R. Popovtzer, A. Agrawal, N. A. Kotov, A. Popovtzer, J. Balter, T. E. Carey and R. Kopelman, Nano Lett., 2008, 8, 4593–4596 CrossRef CAS PubMed.
- B. Zhou, L. Zheng, C. Peng, D. Li, J. Li, S. Wen, M. Shen, G. Zhang and X. Shi, ACS Appl. Mater. Interfaces, 2014, 6, 17190–17199 CAS.
- M. Nahrendorf, H. Zhang, S. Hembrador, P. Panizzi, D. E. Sosnovik, E. Aikawa, P. Libby, F. K. Swirski and R. Weissleder, Circulation, 2008, 117, 379–387 CrossRef CAS PubMed.
- M. Gabriel, C. Decristoforo, D. Kendler, G. Dobrozemsky, D. Heute, C. Uprimny, P. Kovacs, E. Von Guggenberg, R. Bale and I. J. Virgolini, J. Nucl. Med., 2007, 48, 508–518 CrossRef CAS PubMed.
- J. Kim, H. S. Kim, N. Lee, T. Kim, H. Kim, T. Yu, I. C. Song, W. K. Moon and T. Hyeon, Angew. Chem., Int. Ed., 2008, 47, 8438–8441 CrossRef CAS PubMed.
- D. K. Chatteriee, A. J. Rufalhah and Y. Zhang, Biomaterials, 2008, 29, 937–943 CrossRef PubMed.
- J.-H. Lee, Y.-M. Huh, Y.-W. Jun, J.-W. Seo, J.-T. Jang, H.-T. Song, S. Kim, E.-J. Cho, H.-G. Yoon, J.-S. Suh and J. Cheon, Nat. Med., 2007, 13, 95–99 CrossRef CAS PubMed.
- P. Baptista, E. Pereira, P. Eaton, G. Doria, A. Miranda, I. Gomes, P. Quaresma and R. Franco, Anal. Bioanal. Chem., 2008, 391, 943–950 CrossRef CAS PubMed.
- G. Liu, Y.-Y. Lin, J. Wang, H. Wu, C. M. Wai and Y. Lin, Anal. Chem., 2007, 79, 7644–7653 CrossRef CAS PubMed.
- Y. Liu, H. Miyoshi and M. Nakamura, Int. J. Cancer, 2007, 120, 2527–2537 CrossRef CAS PubMed.
- J.-H. Kim, D. A. Heller, H. Jin, P. W. Barone, C. Song, J. Zhang, L. J. Trudel, G. N. Wogan, S. R. Tannenbaum and M. S. Strano, Nat. Chem., 2009, 1, 473–481 CrossRef CAS PubMed.
- S. Wen, K. Li, H. Cai, Q. Chen, M. Shen, Y. Huang, C. Peng, W. Hou, M. Zhu, G. Zhang and X. Shi, Biomaterials, 2013, 34, 1570–1580 CrossRef CAS PubMed.
- J. Xie, G. Liu, H. S. Eden, H. Ai and X. Chen, Acc. Chem. Res., 2011, 44, 883–892 CrossRef CAS PubMed.
- H. Yang, Y. Zhuang, Y. Sun, A. Dai, X. Shi, D. Wu, F. Li, H. Hu and S. Yang, Biomaterials, 2011, 32, 4584–4593 CrossRef CAS PubMed.
- H. Cai, K. Li, J. Li, S. Wen, Q. Chen, M. Shen, L. Zheng, G. Zhang and X. Shi, Small, 2015, 11, 4584–4593 CrossRef CAS PubMed.
- C. Alric, J. Taleb, G. Le Duc, C. Mandon, C. Billotey, A. Le Meur-Herland, T. Brochard, F. Vocanson, M. Janier, P. Perriat, S. Roux and O. Tillement, J. Am. Chem. Soc., 2008, 130, 5908–5915 CrossRef CAS PubMed.
- S. Zhou, Z. Wu, X. Chen, L. Jia and W. Zhu, ACS Appl. Mater. Interfaces, 2014, 6, 11459–11469 CAS.
- W. J. M. Mulder, G. J. Strijkers, G. A. F. van Tilborg, A. W. Griffioen and K. Nicolay, NMR Biomed., 2006, 19, 142–164 CrossRef CAS PubMed.
- J. Y. Park, M. J. Baek, E. S. Choi, S. Woo, J. H. Kim, T. J. Kim, J. C. Jung, K. S. Chae, Y. Chang and G. H. Lee, ACS Nano, 2009, 3, 3663–3669 CrossRef CAS PubMed.
- D. Bonn, H. Kellay, H. Tanaka, G. Wegdam and J. Meunier, Langmuir, 1999, 15, 7534–7536 CrossRef CAS.
- D. Bonn, S. Tanase, B. Abou, H. Tanaka and J. Meunier, Phys. Rev. Lett., 2002, 89, 015701 CrossRef PubMed.
- P. Mongondry, J. F. Tassin and T. Nicolai, J. Colloid Interface Sci., 2005, 283, 397–405 CrossRef CAS PubMed.
- D. W. Thompson and J. T. Butterworth, J. Colloid Interface Sci., 1992, 151, 236–243 CrossRef CAS.
- S. Wang, Y. Wu, R. Guo, Y. Huang, S. Wen, M. Shen, J. Wang and X. Shi, Langmuir, 2013, 29, 5030–5036 CrossRef CAS PubMed.
- Y. Li, D. Maciel, H. Tomas, J. Rodrigues, H. Ma and X. Shi, Soft Matter, 2011, 7, 6231–6238 RSC.
- S. Wang, F. Zheng, Y. Huang, Y. Fang, M. Shen, M. Zhu and X. Shi, ACS Appl. Mater. Interfaces, 2012, 4, 6393–6401 CAS.
- L. Ding, Y. Hu, Y. Luo, J. Zhu, Y. Wu, Z. Yu, X. Cao, C. Peng, X. Shi and R. Guo, Biomater. Sci., 2016, 4, 474–482 RSC.
- R. Mustafa, Y. Hu, J. Yang, J. Chen, H. Wang, G. Zhang and X. Shi, RSC Adv., 2016, 57490–57496 RSC.
- Y. Wu, R. Guo, S. Wen, M. Shen, M. Zhu, J. Wang and X. Shi, J. Mater. Chem. B, 2014, 2, 7410–7418 RSC.
- G. Chen, D. Li, J. Li, X. Cao, J. Wang, X. Shi and R. Guo, New J. Chem., 2015, 39, 2847–2855 RSC.
- R. Mustafa, Y. Luo, Y. Wu, R. Guo and X. Shi, Nanomaterials, 2015, 5, 1716–1731 CrossRef CAS.
- P. A. Wheeler, J. Z. Wang, J. Baker and L. J. Mathias, Chem. Mater., 2005, 17, 3012–3018 CrossRef CAS.
- I. Grabchev, C. Petkov and V. Bojinov, Dyes Pigm., 2004, 62, 229–234 CrossRef CAS.
- Z. Nazarpoor, S. Ma, P. T. Fanson, O. S. Alexeev and M. D. Amiridis, Polym. Degrad. Stab., 2012, 97, 439–451 CrossRef CAS.
- B. Zhou, Z. Xiong, J. Zhu, M. Shen, G. Tang, C. Peng and X. Shi, Nanomedicine, 2016, 11, 1639–1652 CrossRef CAS PubMed.
- C. Peng, K. Li, X. Cao, T. Xiao, W. Hon, L. Zheng, R. Guo, M. Shen, G. Zhang and X. Shi, Nanoscale, 2012, 4, 6768–6778 RSC.
- C. Peng, L. Zheng, Q. Chen, M. Shen, R. Guo, H. Wang, X. Cao, G. Zhang and X. Shi, Biomaterials, 2012, 33, 1107–1119 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra18718h |
| ‡ Rania Mustafa, Benqing Zhou, JiaYang contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |




