Design and synthesis of sugar-benzohydrazides: low molecular weight organogelators†
Abstract
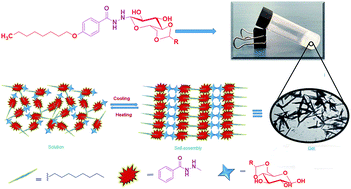
A novel class of methyltriglycol benzohydrazide based N-glycosylamines containing long alkyl chain derivatives were synthesized in good yield and characterized using NMR (1H and 13C) spectral analysis. The gelation test shows that these derivatives form gels with a wide range of polar (1,2-dichlorobenzene, chloroform) and non-polar (1,2-dichloroethane, toluene, benzene) solvents even with lower CGC (1.0%). Morphological studies of these amphiphilic glycosidic gels were studied using FE-SEM, TEM and powder XRD analysis. Studies further show the formation of 3D self-assembled spherical and rod structures which is due to the influence of organic solvents. It is found that hydrogen bonding and van der Waals interactions play an important role in the self-assembled structure.

 Please wait while we load your content...
Please wait while we load your content...