New one-pot method for the synthesis of pyrrolidinofullerenes†
Abstract
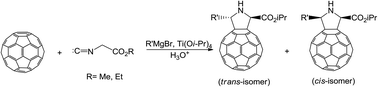
The reaction of fullerene C60 with isocyanoacetates and EtMgBr in the presence of stoichiometric amounts of Ti(Oi-Pr)4 was studied for the first time. Unlike esters and nitriles of carboxylic acids and isonitriles, isocyanoacetates were found to react with C60 under the developed conditions to give N-unsubstituted pyrrolidinofullerenes. The electrochemical reduction of the C60 derivatives we synthesized was found to proceed less easily than that of C60 but more easily than that of unsubstituted pyrrolidinofullerenes.

 Please wait while we load your content...
Please wait while we load your content...