Pyridyl-phenylethynylene bis-urea macrocycles: self-assembly and utility as a nanoreactor for the selective photoreaction of isoprene†
Sahan R. Salpage,
Yuewen Xu,
Bozumeh Som,
Ammon J. Sindt,
Mark D. Smith and
Linda S. Shimizu *
*
Department of Chemistry and Biochemistry, University of South Carolina, 631 Sumter St., Columbia, SC 29208, USA. E-mail: shimizuls@mailbox.sc.edu; Fax: +1-803-777-9521; Tel: +1-803-777-2066
First published on 6th October 2016
Abstract
Porous organic crystalline materials were obtained by the self-assembly of pyridyl-phenylethynylene bis-urea macrocycles (1). In contrast to the typical columnar assembly of other bis-urea macrocycles, 1 assembled into interdigitated layers to afford a host with small 1D channels with a pore diameter of ∼4.5 Å. Herein, we discuss the origin between the expected and observed assembly and demonstrate the utility of these activated organic crystals to absorb a guest and maintain crystallinity during the reaction of the encapsulated guest and subsequent product isolation. Isoprene was chosen as a model monomer due to the small channel diameter of 1. The activated crystals were able to absorb isoprene and facilitate a photoinitiated and stereoselective oligomerization to produce trans-1,4-polyisoprene with low dispersity. The products were readily extracted from the crystals and analysis of the microstructure of the oligomer showed 97% trans-1,4 content and a dispersity (Đ) of 1.39. Despite the altered assembly pattern of this bis-urea macrocycle, powder X-ray diffraction studies demonstrated that the crystalline host was remarkably robust and stable throughout the activation, isoprene absorption, photoinduced polymerization, and product recovery processes. Our future investigations are focused on assessing the stability of the isoprene radicals within the host and evaluating the loading and reaction of other monomers.
1 Introduction
Porous materials have demonstrated utility in catalysis, in storage and molecular separation as well as for emerging technology in energy and medicine.1–4 Recently, porous materials have also been used to trap molecules for facile X-ray structure determination.5–8 Our interests lie in identifying simple organic building blocks that assemble with high fidelity to give functional porous structures and to utilize these crystalline materials to probe reactions in confined environments. Indeed, confined media including crystals, inclusion complexes, microporous zeolites, coordination polymers and mesoporous materials have been used to perform selective organic reactions such as topochemically driven inclusion polymerization reactions.9–14 For example narrow channels of urea, thiourea, deoxycholic acid (DCA), and perhydrotriphenylene (PHTP), have been used to polymerize a variety of diene monomers to obtain stereoregular polymers.15Our group employs well-defined porous materials from self-assembled bis-urea macrocycles building blocks that consist of two urea groups and two C-shaped spacers (Fig. 1a). When the urea groups are preorganized approximately perpendicular to the plane of the macrocycle and in the absence of competing hydrogen bond acceptors, these macrocycles assemble into columnar structures.16 The phenylethynylene bis-urea 2 assembled into columns of ∼9 Å in diameter (Fig. 1c), affording functional crystals that were applied to the photodimerizations of coumarins, chromones and acenaphthylene.17–19 Here, we replace the central aryl group of 2 with the pyridyl unit to test the effects of the heterocycle on the subsequent assembly (Fig. 1b). We then demonstrate the utility of these porous crystals for guest absorption as well as for the subsequent photoreaction of isoprene within this confined nanoreactor.
 | ||
| Fig. 1 Heteroatoms, in the form of a pyridyl nitrogen in 1, altered the typically columnar assembly of bis-urea macrocycles. The interdigitated assembly of this new material afforded robust crystals with smaller 1D channels, which are used to polymerize isoprene to produce trans-1,4-polyisoprene. (a) Schematic representation of macrocycle 1 and 2. (b) Interdigitated assembly of 1 forms 1D channels with a diameter of 4.5 Å. (c) Columnar assembly of 2 forms 1D channels with a diameter of 9 Å.19 (d) Photo irradiation of isoprene in host crystals produces trans-1,4-polyisoprene in high selectivity. | ||
2 Experimental
2.1 General
All chemicals were purchased from Sigma-Aldrich or VWR and used without further purification. Triazinanone was prepared as previously described.20 1H-NMR and 13C-NMR spectra were recorded on Varian Mercury/VX300 or VX400. PXRD data was collected on Rigaku Dmax 2200 powder X-ray diffractometer using Cu Kα radiation. The step-scans were collected at +0.05° steps at an angular range of 2–20° 2θ at ambient conditions. TGA data were collected on TA SDT Q600. UV irradiations were performed in a Rayonet reactor equipped with 16 × 120 W lamps (350 nm). GPC data were collected using Varian 290-Lc equipped with a RI detector in THF using polystyrenes as the standard.2.2 Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH, 95
MeOH, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). The product was further washed with water followed by hexane to remove excess TBAF and dried in vacuo to obtain the diol as pale yellow solid 1.3 g, (91%); mp 221 °C; 1H-NMR: (400 MHz, DMSO-d6 δ): 7.93–7.89 (t, 1H, J = 8.1 Hz, Ar-H), 7.65 (d, J = 7.8 Hz, 2H, Ar-H), 7.60 (d, 4H, J = 8.1 Hz, Ar-H), 7.41 (d, J = 8.1 Hz, 4H, Ar-H), 5.35 (t, J = 5.8 Hz, 2H, –OH), 4.55 (d, J = 5.7 Hz, 4H, –CH2); 13C-NMR: (100 MHz, DMSO-d6): δ 145.16, 143.48, 138.45, 132.30, 127.39, 127.33, 119.89, 89.82, 88.59, 63.13; IR (cm−1): 3340, 3315, 2214, 1440, 1244, 1163, 804; HRMS (EI+): [M+] calculated: 339.1259, found: 339.1260.
5). The product was further washed with water followed by hexane to remove excess TBAF and dried in vacuo to obtain the diol as pale yellow solid 1.3 g, (91%); mp 221 °C; 1H-NMR: (400 MHz, DMSO-d6 δ): 7.93–7.89 (t, 1H, J = 8.1 Hz, Ar-H), 7.65 (d, J = 7.8 Hz, 2H, Ar-H), 7.60 (d, 4H, J = 8.1 Hz, Ar-H), 7.41 (d, J = 8.1 Hz, 4H, Ar-H), 5.35 (t, J = 5.8 Hz, 2H, –OH), 4.55 (d, J = 5.7 Hz, 4H, –CH2); 13C-NMR: (100 MHz, DMSO-d6): δ 145.16, 143.48, 138.45, 132.30, 127.39, 127.33, 119.89, 89.82, 88.59, 63.13; IR (cm−1): 3340, 3315, 2214, 1440, 1244, 1163, 804; HRMS (EI+): [M+] calculated: 339.1259, found: 339.1260.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes, 1
hexanes, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain the dibromide as pale yellow solid 0.9 g, (66%); mp 183 °C; 1H-NMR: (400 MHz, CD3Cl δ): 7.75–7.64 (t, J = 8.2 Hz, 1H, Ar-H), 7.58 (d, J = 7.2 Hz, 4H, Ar-H), 7.48 (d, 2H, J = 7.8 Hz, Ar-H), 7.39 (d, J = 8.2 Hz, 4H, Ar-H), 4.49 (s, 4H, –CH2); 13C-NMR: (100 MHz, CD3Cl) δ: 143.88, 138.91, 136.75, 132.72, 129.36, 126.66, 122.42, 89.32, 89.20, 33.04; IR (cm−1): 3315, 2214, 1440, 1244, 1163, 804; HRMS (EI+): [M+] calculated: 462.9571, found: 462.9590.
1) to obtain the dibromide as pale yellow solid 0.9 g, (66%); mp 183 °C; 1H-NMR: (400 MHz, CD3Cl δ): 7.75–7.64 (t, J = 8.2 Hz, 1H, Ar-H), 7.58 (d, J = 7.2 Hz, 4H, Ar-H), 7.48 (d, 2H, J = 7.8 Hz, Ar-H), 7.39 (d, J = 8.2 Hz, 4H, Ar-H), 4.49 (s, 4H, –CH2); 13C-NMR: (100 MHz, CD3Cl) δ: 143.88, 138.91, 136.75, 132.72, 129.36, 126.66, 122.42, 89.32, 89.20, 33.04; IR (cm−1): 3315, 2214, 1440, 1244, 1163, 804; HRMS (EI+): [M+] calculated: 462.9571, found: 462.9590.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl ether
ethyl ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH, 4
MeOH, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) to obtain the product as pale yellow solid 0.19 g (19%); 1H-NMR: (300 MHz, CDCl3 δ): 7.70–7.61 (m, 2H, Ar-H), 7.63–7.57 (m, 8H, Ar-H), 7.47–7.45 (m, 4H, Ar-H), 7.36–7.34 (m, 8H, Ar-H), 4.59 (s, 8H, –CH2–), 4.25 (s, 8H, –CH2–), 0.96 (s, 18H, –CH3); 13C-NMR: (100 MHz, CDCl3 δ): 155.87, 143.87, 139.46, 136.40, 132.50, 128.15, 125.96, 121.16, 89.45, 88.49, 61.82, 54.38, 48.59, 28.24; IR (cm−1): 3315, 2214, 1630, 1440, 1244, 1163, 804; HRMS (ES+): [M + H]+ calculated: 921.4604, found: 921.4582.
0.5) to obtain the product as pale yellow solid 0.19 g (19%); 1H-NMR: (300 MHz, CDCl3 δ): 7.70–7.61 (m, 2H, Ar-H), 7.63–7.57 (m, 8H, Ar-H), 7.47–7.45 (m, 4H, Ar-H), 7.36–7.34 (m, 8H, Ar-H), 4.59 (s, 8H, –CH2–), 4.25 (s, 8H, –CH2–), 0.96 (s, 18H, –CH3); 13C-NMR: (100 MHz, CDCl3 δ): 155.87, 143.87, 139.46, 136.40, 132.50, 128.15, 125.96, 121.16, 89.45, 88.49, 61.82, 54.38, 48.59, 28.24; IR (cm−1): 3315, 2214, 1630, 1440, 1244, 1163, 804; HRMS (ES+): [M + H]+ calculated: 921.4604, found: 921.4582.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 20% [NH(CH2CH2OH)2/H2O, adjusted with HCl to pH ∼ 2]: MeOH and heated to reflux for 48 h. A pale yellow precipitate formed after 24 h. The solution was cooled in an ice bath for 30 min. The product was suction filtered, washed with H2O (30 mL) and MeOH (30 mL), and dried in vacuo to obtain the final product as pale yellow powder 0.14 g (90%). 1H-NMR: (400 MHz, DMSO-d6 δ): 7.91–7.87 (m, 2H, Ar-H), 7.64–7.60 (m, 12H, Ar-H), 7.33–7.31 (m, 8H, Ar-H), 6.69 (t, J = 6.2 Hz, 4H, –NH), 4.30 (d, J = 6.1 Hz, 8H, –CH2); 13C-NMR: (100 MHz, DMSO-d6 δ): 158.54, 143.84, 143.29, 138.07, 132.28, 127.53, 127.09, 119.60, 89.48, 88.50, 43.01; IR (cm−1): 3315, 2214, 1630, 1554, 1440, 1244, 1163, 804; HRMS (ES+): [M + H]+ calculated: 727.2821, found: 727.2795.
1 mixture of 20% [NH(CH2CH2OH)2/H2O, adjusted with HCl to pH ∼ 2]: MeOH and heated to reflux for 48 h. A pale yellow precipitate formed after 24 h. The solution was cooled in an ice bath for 30 min. The product was suction filtered, washed with H2O (30 mL) and MeOH (30 mL), and dried in vacuo to obtain the final product as pale yellow powder 0.14 g (90%). 1H-NMR: (400 MHz, DMSO-d6 δ): 7.91–7.87 (m, 2H, Ar-H), 7.64–7.60 (m, 12H, Ar-H), 7.33–7.31 (m, 8H, Ar-H), 6.69 (t, J = 6.2 Hz, 4H, –NH), 4.30 (d, J = 6.1 Hz, 8H, –CH2); 13C-NMR: (100 MHz, DMSO-d6 δ): 158.54, 143.84, 143.29, 138.07, 132.28, 127.53, 127.09, 119.60, 89.48, 88.50, 43.01; IR (cm−1): 3315, 2214, 1630, 1554, 1440, 1244, 1163, 804; HRMS (ES+): [M + H]+ calculated: 727.2821, found: 727.2795.2.3 X-ray diffraction
Table 1| 1 | 3 | |
|---|---|---|
| Empirical formula | C48H34N6O2 | C61H58Cl2N8O2 |
| Formula weight | 726.81 | 1006.05 |
| Temperature/K | 100(2) | 150(2) |
| Crystal system | Orthorhombic | Monoclinic |
| Space group | Pbcn | P21/m |
| a/Å | 19.711(5) | 9.6662(4) |
| b/Å | 8.983(2) | 18.8900(8) |
| c/Å | 26.520(6) | 14.4567(6) |
| α/° | 90 | 90 |
| β/° | 90 | 91.464(1) |
| γ/° | 90 | 90 |
| Volume/Å3 | 4695.7(18) | 2638.85(19) |
| Z | 4 | 2 |
| ρcalc (g cm−3) | 1.028 | 1.266 |
| μ/mm−1 | 0.064 | 0.175 |
| F(000) | 1520 | 1060 |
| Crystal size/mm3 | 0.48 × 0.20 × 0.08 | 0.28 × 0.20 × 0.08 |
| 2θ range/° | 3.072–45.078 | 1.41–23.26 |
| Index ranges | −21 ≤ h ≤ 21 | −10 ≤ h ≤ 10 |
| −9 ≤ k ≤ 9 | −20 ≤ k ≤ 20 | |
| −28 ≤ l ≤ 28 | −16 ≤ l ≤ 16 | |
| Reflections collected | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 741 741 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 637 637 |
| Independent reflections | 3087 | 3930 |
| Rint | 0.1417 | 0.0615 |
| Parameters | 261 | 379 |
| GoF on F2 | 1.080 | 1.017 |
| Final R1 [I > 2σ(I)] | 0.0596 | 0.0750 |
| Final wR2 [I > 2σ(I)] | 0.1732 | 0.2161 |
| Final R1 [all data] | 0.0909 | 0.1066 |
| Final wR2 [all data] | 0.1868 | 0.2410 |
| Δρmin,max/e Å−3 | 0.20/−0.15 | 0.822/−0.321 |
3 Results and discussion
The bis-urea macrocycle was synthesized in four steps from commercially available 2,6-dibromopyridine using a protected urea strategy (Scheme 1). Macrocycle 3 afforded pale yellow crystals suitable for X-ray diffraction by slow evaporation from CH2Cl2 to give the solvate (C60H56N8O2·CH2Cl2 monoclinic, space group P21/m). Scheme 1 shows a view from the X-ray structure of 3 (ball and stick view). The two urea groups in macrocycle 3 show a parallel arrangement with the pyridyl rings pointing in roughly the opposite direction. More typically in bis-urea macrocycles, the urea groups are aligned anti-parallel, presumably to minimize the dipole moment.16Following deprotection of 3, host 1 (20 mg/4 mL DMSO) was crystallized by vapor diffusion of methanol affording pale yellow needle crystals suitable for single crystal X-ray diffraction. The crystal structure revealed the expected bis-urea macrocycle as a solvate; however, 1 was not planar but folded into a bowl or saddle conformation with C2 point symmetry (Fig. 2a). Here, the two urea groups remain oriented in the same direction. This folded architecture assembles through typical bifurcated urea hydrogen bonds (N(H)⋯O distances of 2.81–2.87 Å) with four neighboring macrocycles to afford 2D assemblies of interdigitated cycles (Fig. 2b). The packing of the layers creates tubular channels of ∼4.5 Å in diameter along the crystallographic b axis (Fig. 2c). The channels are occupied by disordered solvent molecules (DMSO and/or MeOH). Adjacent layers alternate ureas in an anti-parallel fashion resulting in a cancellation of the dipoles. The assembly is further stabilized by aryl stacking and CH-pi interactions. The crystalline structures have regular and aligned 1D pores with diameters of ∼4.5 Å (Fig. S9, ESI†).
The conformational difference between the saddle structure of 1 (X = N) with the relatively planar conformation of the carbon derivative 2 (X = CH), reported previously,19 is striking and appears to drive the interdigitated assembly of 1 over the columnar assembly of 2. The density of 1, calculated in the absence of the disordered guests, is 1.028 mg mm−3 (orthorhombic, space group Pbcn) with a solvent accessible volume per unit cell estimated as 1341.4 Å3 (28.6% of the total unit cell volume). In comparison columnar assembled 2 (monoclinic, space group P21/n) has a calculated solvent-free density of 1.061 mg mm−3 with a solvent-accessible volume of the unit cell estimated as 491.1 Å3 (21.6% of the total unit cell volume).19 We further compared the assemblies using Hirshfeld analysis.22–24 Interestingly, the two assembly motifs show similar contributions of key interactions including hydrogen bonding (1: 5.5% vs. 2: 6.1% O⋯H), and CH-aryl interactions (1: 26.5% vs. 2: 26.4% C⋯H contacts) but displayed small differences in aryl stacking interactions (1: 7% vs. 2: 11.7% C⋯C) and in contacts to nitrogen (1: 4.3% vs. 2: 1.4% N⋯H) (Fig. S11–S12, ESI†). A screen of crystallization conditions has not yet yielded other crystal forms of 1.
The smaller diameter channels of 1 versus 2 (4.5 vs. 9 Å) are comparable to channels that encapsulate isoprene in inclusion complexes with dipeptides or cyclotriphosphazenes.25,26 Isoprene and its polymers are widely used in industry to synthesize block copolymers,27–29 as compatibilizers for natural rubber and acrylic polymer blends,30,31 as nanocomposites,32,33 and to produce macromolecular core shell nano architectures.34,35 These materials can have low glass transition temperatures and unsaturated backbone or side chains that allow further functionalization.36 Thus, isoprene provides an interesting model monomer to evaluate the stability of host 1 as a container to reversibly absorb a guest and to remain stable over the course of the reaction of the encapsulated material. Conventional polymerization of isoprene provides isomeric polymers through different addition modes (cis-1,4-, trans-1,4, 1,2- or 3,4-) depending upon how the C–C double bonds react (Fig. 3a). Anionic polymerization is the technique of choice but requires stringent reaction conditions and leads to low trans-1,4-selectivity (Table S1, ESI†).37 The trans-1,4 (Balata) is produced by plants using enzymatic synthesis. It exhibits excellent thermoplastic characteristics, high tensile strength, abrasion resistance, and is free from odor and taste.38,39 Yet, stereoselective synthesis of trans-1,4-polyisoprene remains challenging. Confined environments have been applied for the selective radical polymerization of isoprene trapped within the controlled pores, a process that typically requires high energy gamma irradiation necessitating careful handling and specialized reactors.15
Our goal was to evaluate if encapsulation in the small channels of 1 could modulate the reactivity of isoprene and enable polymerization under mild conditions. Initially, the channels of 1 are filled with solvent, which was removed prior to the introduction of isoprene. Thermogravimetric analysis (TGA) showed a two step desorption from rt to 250 °C with a 9.98% weight loss. The crystals were also heated at 120 °C for 3 h resulting in a similar weight loss (Fig. S13, ESI†). Powder X-ray diffraction (PXRD) was used to compare the solvated and activated structures before and after solvent removal (Fig. 3c, patterns ii and iii). Comparison of the two PXRD patterns show they are nearly identical suggesting that the material still maintains its crystallinity after solvent evacuation. Indeed, removal of the disordered solvent did not change the morphology of the crystals.
Freshly recrystallized 1 (20 mg) was heated at 120 °C to remove the disordered solvent and further evacuated under high vacuum before exposure to isoprene under reduced pressure at room temperature for 3 h. A custom made loading apparatus was used to absorb isoprene from its vapor phase under reduced pressure at room temperature for 24 h, conditions in order to approach equilibrium (Fig. 3b). A sample of the crystals (2 mg) was dissolved in DMSO-d6 and analysed by 1H NMR. Comparison of the integrated peaks suggests that isoprene had been absorbed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host
1 host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest ratio (Fig. S19†). The host 1·isoprene material was frozen in liquid N2, vacuum, sealed, and UV-irradiated in a Rayonet RPR-200 UV reactor equipped with 350 nm lamps for 24 h at rt. The irradiated material showed similar PXRD pattern suggesting that the crystal form was not altered during the loading process and the subsequent reaction (Fig. 3c, patterns iii and iv). The polymer was then extracted from the host by sonication with CHCl3 (10 mL). The suspension of host 1 and polymer was filtered to recover the host and the filtrate concentrated in vacuo. Polyisoprene was precipitated by the dropwise addition of ice-cold methanol.
guest ratio (Fig. S19†). The host 1·isoprene material was frozen in liquid N2, vacuum, sealed, and UV-irradiated in a Rayonet RPR-200 UV reactor equipped with 350 nm lamps for 24 h at rt. The irradiated material showed similar PXRD pattern suggesting that the crystal form was not altered during the loading process and the subsequent reaction (Fig. 3c, patterns iii and iv). The polymer was then extracted from the host by sonication with CHCl3 (10 mL). The suspension of host 1 and polymer was filtered to recover the host and the filtrate concentrated in vacuo. Polyisoprene was precipitated by the dropwise addition of ice-cold methanol.
The products displayed the simple 1H NMR spectra shown in Fig. 4a. The polymer microstructure consists mainly of trans-1,4-polyisoprene in 96.7% with 3.3% cis-1,4-isomer. The absence of signals at ∼5.9 ppm and ∼4.7 ppm indicated that no significant amount of the branched 1,2- or 3,4-structures had been formed. Gel permeation chromatography was used to analyse the molar mass of the resulting polymer. The polymer shows a Mn = 4400 g mol−1 with a dispersity (Đ) of 1.39 (Fig. 4b). The average length of the channels in the bulk material was estimated from dark field microscope images as ∼212.8 μm. Assuming isoprene is fully absorbed in channels of 1, the rough maximum Mw is ∼3.02 × 107 g mol−1. Studies are underway to optimize conditions for the polymerization. Evacuated host 2 (20 mg) was exposed to isoprene and similarly UV-irradiated; however, no oligomers or polymers were formed, which suggests that isoprene either has a low affinity for the larger channels of 2 or is not reactive within these channels.
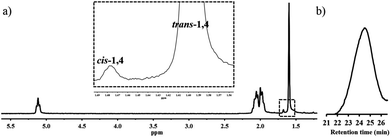 | ||
| Fig. 4 Characterization of the isolated polyisoprene. (a) 1H NMR (CDCl3, 400 MHz) of the polyisoprene. (b) GPC trace of polyisoprene in THF. | ||
Isoprene has a low boiling point and likely desorbs from these hosts at room temperature over the 24 h irradiation period. Thus, full characterization of the host·isoprene material was not possible and one cannot rule out the possibility that this is a simple mixture. However, the reasonably high molecular weight polymer and low dispersity for a free radical polymerization process induced by UV-irradiation of host 1·isoprene material is a strong indication that isoprene is bound within the channels of 1 and that the reaction proceeds relatively quickly before isoprene has a chance to desorb. Recent work from Kitagawa and co-workers on the radical polymerization of 2,3-dimethyl-1,3-butadiene in a porous coordination polymer suggests that their confined environment stabilizes the propagating radicals and inhibits radical termination.40
4 Conclusions
In conclusion, substitution of a pyridyl group for a benzene markedly altered the assembly of a bis-urea macrocycle, favouring interdigitated over columnar assembly. While this new structure was still organized through typical three centered urea hydrogen bonds, it displayed enhanced contacts with nitrogen, slightly less aryl stacking interactions, and a significantly smaller channel size. Despite its small pore, the activated material was able to absorb isoprene and facilitate its polymerization by mild UV-irradiation. The trans-1,4-polyisoprene was obtained in high selectivity (96.7% trans content by NMR). The observed PDI of 1.39 was considerably low for a photo-induced free radical polymerization and coupled with the high selectivity experimentally suggests that the polymerization occurs within the confined 1D channels of 1. The resulting polymer was easily released from the host by sonication in CHCl3, and the crystalline host was recovered by filtration and reused. The structure of the host was remarkably robust and stable throughout the activation process, isoprene loading, polymerization and recovery. Current studies are focused on screening for different crystal forms of 1 as well as on optimizing the degree of polymerization for this ready and selectivity synthesis of stereoregular trans-1,4-polyisoprene. Alternative methods for extruding the polyisoprene from the crystalline nanoreactor could potentially align the polymer chains and further modulate the material properties. Future studies will apply this new bis-urea host as confined media to control the tacticity of polymers of other vinyl monomers.Acknowledgements
The authors gratefully acknowledge partial support for this work from the NSF (CHE-1608874 CHE-1305136) and by the University of South Carolina, (SPARC 13020E-150) for SRS. We thank Prof. Brian Benicewicz and Yucheng Huang for helpful discussions and GPC analysis.Notes and references
- E. Sanna, E. C. Escudero-Adán, C. López, P. Ballester, C. Rotger and A. Costa, J. Org. Chem., 2016, 81, 5173 CrossRef CAS PubMed.
- A. G. Slater and A. I. Cooper, Science, 2015, 348, 988 CrossRef CAS PubMed.
- J. T. A. Jones, T. Hasell, X. Wu, J. Bacsa, K. E. Jelfs, M. Schmidtmann, S. Y. Chong, D. J. Adams, A. Trewin, F. Schiffman, F. Cora, B. Slater, A. Steiner, G. M. Day and A. I. Cooper, Nature, 2011, 474, 367 CrossRef CAS PubMed.
- J. R. Holst, A. Trewin and A. I. Cooper, Nat. Chem., 2010, 2, 915 CrossRef CAS PubMed.
- S. Urban, R. Brkljača, M. Hoshino, S. Lee and M. Fujita, Angew. Chem., Int. Ed., 2016, 55, 2678 CrossRef CAS PubMed.
- Y. Inokuma, T. Ukegawa, M. Hoshino and M. Fujita, Chem. Sci., 2016, 7, 3910 RSC.
- E. Sanna, E. C. Escudero-Adan, A. Bauza, P. Ballester, A. Frontera, C. Rotger and A. Costa, Chem. Sci., 2015, 6, 5466 RSC.
- Y. Inokuma, S. Yoshioka, J. Ariyoshi, T. Arai, Y. Hitora, K. Takada, S. Matsunaga, K. Rissanen and M. Fujita, Nature, 2013, 495, 461 CrossRef CAS PubMed.
- A. Comotti, S. Bracco, M. Mauri, S. Mottadelli, T. Ben, S. Qiu and P. Sozzani, Angew. Chem., Int. Ed., 2012, 51, 10136 CrossRef CAS PubMed.
- T. Uemura, N. Yanai and S. Kitagawa, Chem. Soc. Rev., 2009, 38, 1228 RSC.
- Y. Lu, Y. Yang, A. Sellinger, M. Lu, J. Huang, H. Fan, R. Haddad, G. Lopez, A. R. Burns, D. Y. Sasaki, J. Shelnutt and C. J. Brinker, Nature, 2001, 410, 913 CrossRef CAS PubMed.
- K. Tajima and T. Aida, Chem. Commun., 2000, 2399 RSC.
- K. Kageyama, J.-i. Tamazawa and T. Aida, Science, 1999, 285, 2113 CrossRef CAS PubMed.
- C.-G. Wu and T. Bein, Science, 1994, 266, 1013 CAS.
- P. A. Gale and J. W. Steed, Supramolecular chemistry; from molecules to nanomaterials, Reactions in Solid-State Inclusion Compounds, ed. K. D. M. Harris, B. A. Palmer and G. R. Edwards-Gau, Wiley, West Sussex, 2012, vol. 4, pp. 1589–1612 Search PubMed.
- L. S. Shimizu, S. R. Salpage and A. A. Korous, Acc. Chem. Res., 2014, 47, 2116 CrossRef CAS PubMed.
- S. R. Salpage, L. S. Donevant, M. D. Smith, A. Bick and L. S. Shimizu, J. Photochem. Photobiol., A, 2016, 315, 14 CrossRef CAS.
- S. Dawn, S. R. Salpage, B. A. Koscher, A. Bick, A. C. Wibowo, P. J. Pellechia and L. S. Shimizu, J. Phys. Chem. A, 2014, 118, 10563 CrossRef CAS PubMed.
- S. Dawn, M. B. Dewal, D. Sobransingh, M. C. Paderes, A. C. Wibowo, M. D. Smith, J. A. Krause, P. J. Pellechia and L. S. Shimizu, J. Am. Chem. Soc., 2011, 133, 7025 CrossRef CAS PubMed.
- A. R. Mitchell, P. F. Pagoria, C. L. Coon, E. S. Jessop, J. F. Poco, C. M. Tarver, R. D. Breithaupt and G. L. Moody, Propellants, Explos., Pyrotech., 1994, 19, 232 CrossRef CAS.
- Y. Liang, Y.-X. Xie and J.-H. Li, J. Org. Chem., 2006, 71, 379 CrossRef CAS PubMed.
- M. A. Spackman, J. J. McKinnon and D. Jayatilaka, CrystEngComm, 2008, 10, 377 CAS.
- A. Parkin, G. Barr, W. Dong, C. J. Gilmore, D. Jayatilaka, J. J. McKinnon, M. A. Spackman and C. C. Wilson, CrystEngComm, 2007, 9, 648 RSC.
- J. J. McKinnon, D. Jayatilaka and M. A. Spackman, Chem. Commun., 2007, 3814 RSC.
- G. Distefano, A. Comotti, S. Bracco, M. Beretta and P. Sozzani, Angew. Chem., Int. Ed., 2012, 51, 9258 CrossRef CAS PubMed.
- H. R. Allcock, E. N. Silverberg, G. K. Dudley and S. R. Pucher, Macromolecules, 1994, 27, 7550 CrossRef CAS.
- G. Liu, Z. Li and X. Yan, Polymer, 2003, 44, 7721 CrossRef CAS.
- T. Hayakawa, S. Horiuchi, H. Shimizu, T. Kawazoe and M. Ohtsu, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 2406 CrossRef CAS.
- S. C. Schmidt and M. A. Hillmyer, Macromolecules, 1999, 32, 4794 CrossRef CAS.
- J. Wootthikanokkhan and B. Tongrubbai, J. Appl. Polym. Sci., 2003, 88, 921 CrossRef CAS.
- C. Batis, G. Karanikolopoulos, M. Pitsikalis and N. Hadjichristidis, Macromolecules, 2000, 33, 8925 CrossRef CAS.
- M. Knite, V. Teteris, A. Kiploka and J. Kaupuzs, Sens. Actuators, A, 2004, 110, 142 CrossRef CAS.
- J. Ren, A. S. Silva and R. Krishnamoorti, Macromolecules, 2000, 33, 3739 CrossRef CAS.
- C. Cheng, K. Qi, E. Khoshdel and K. L. Wooley, J. Am. Chem. Soc., 2006, 128, 6808 CrossRef CAS PubMed.
- K. S. Murthy, Q. Ma, E. E. Remsen, T. Kowalewski and K. L. Wooley, J. Mater. Chem., 2003, 13, 2785 RSC.
- D. S. Germack and K. L. Wooley, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 4100 CrossRef CAS.
- D. J. Worsfold and S. Bywater, Can. J. Chem., 1964, 42, 2884 CrossRef CAS.
- J.-S. Song, B.-C. Huang and D.-S. Yu, J. Appl. Polym. Sci., 2001, 82, 81 CrossRef CAS.
- E. G. Kent and F. B. Swinney, Ind. Eng. Chem. Prod. Res. Dev., 1966, 5, 134 CrossRef CAS.
- T. Uemura, R. Nakanishi, S. Mochizuki, Y. Murata and S. Kitagawa, Chem. Commun., 2015, 51, 9892 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: NMR spectra, PXRD, TGA, isoprene loading studies, characterization of the polymer and crystallographic data CCDC. CCDC 1457935 and 1463771. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra18681e |
| This journal is © The Royal Society of Chemistry 2016 |



