Crystallization and temperature-dependent structure deflection of C6mimBr ionic liquid intercalated in LAPONITE®†
Fangling Jiang‡
a,
Cheng Li‡a,
Xiaojing Guoa,
Haiying Fua,
Guozhong Wu*a and
Shimou Chen*b
aShanghai Institute of Applied Physics, Chinese Academy of Sciences, Shanghai 201800, China. E-mail: wuguozhong@sinap.ac.cn; Fax: +86-021-39194526; Tel: +86-021-39194526
bInstitute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China. E-mail: chenshimou@ipe.ac.cn; Fax: +86-10-82544875; Tel: +86-10-82544800 Tel: +86-13671151768
First published on 10th October 2016
Abstract
The physicochemical properties of large molecules confined in nanopores are expected to be different from those of the bulk. This study investigates the cation–anion relative position and the molecular orientation of the 1-hexyl-3-methylimidazolium bromide (C6mimBr) ionic liquid intercalated in LAPONITE® by a temperature-dependent X-ray absorption fine structure (XAFS) and some traditional methods (TEM, DSC, XRD, and IR). DSC and TEM analyses revealed the formation of C6mimBr crystals intercalated in LAPONITE®. The XAFS and XRD analyses at ambient temperature revealed that an ordered monolayer structure, with C6mim+ cations intercalated in the interlayer nanospace and Br located on the edge of the LAPONITE®, was formed when ILs were intercalated in LAPONITE®. The results also demonstrated that the enhanced interactions, the formed hydrogen bonds, as well as the ordered monolayer arrangement induced the formation of C6mimBr crystals when intercalated in LAPONITE®. In situ XAFS analysis with a combination of XRD patterns at varied temperatures revealed that the structure orientation of the intercalated C6mim+ cations tends to deflect and maintain the ordered monolayer arrangement with the elevation of temperature. The ordered crystal structure still exists at 120 °C and disappears at a higher temperature.
Introduction
Ionic liquids (ILs) have received much attention due to their importance in a broad range of applications.1,2 These applications stem from their special physical and chemical properties including negligible vapor pressure, high thermal stability, and wide electrochemical window.3 In recent years, ILs immobilized on solid supports have gained increasing attention because these compounds are applied in material expansion.4,5 In order to approach a smart-design for this kind of IL based hybrids, many theoretical and experimental efforts have been made to study the microstructures and phase behavior of ILs immobilized on a solid surface or incorporated into a porous solid. For instance, Sha et al.6 reported the bilayer structure formation of ILs loaded on SiO2 solid surface by atom molecular dynamics simulations. Singh et al.7 found that the Tm (melting point) of imidazolium-based ILs immobilized on a nanoporous silica matrix was depressed significantly compared to bulk ILs. Wang et al.8 investigated the microscopic ionic structures and orientational preferences of imidazolium-based ILs absorbed on chemically different quartz surfaces by atomistic molecular dynamics simulations, concluding that the imidazolium rings lie preferentially perpendicular to Si(OH)2 surface and the anions are mainly absorbed on positively charged SiH2 surface. Zhou et al.9 evaluated the nanoscale interactions of ILs at uncharged graphene and charged mica solid surfaces by using high resolution X-ray interface scattering and fully atomistic molecular dynamics simulations, suggesting that the structure of ILs exhibits a mixed cation/anion layering at uncharged graphene surfaces and an alternating cation/anion layering at the charged mica surface. Gong et al.10 performed atomic force microscopy (AFM) to reveal the extended layering structure of ILs confined to an amorphous carbon surface. Hence, the phase behavior and microstructure of ILs in various supports are relatively different, which will further determine the physicochemical properties of the ILs-based hybrids.LAPONITE® (LAPONITE®) is composed of two silica tetrahedral sheets with a central magnesium octahedral sheet. The special structure of LAPONITE® caused a wide range of applications in paints, cosmetics, and inks.11 The reported LAPONITE® empirical formula is Na0.7+[(Si8Mg5.5Li0.3)O20(OH)4]0.7−. The occurrence of silanol groups at the edges of the sheets is ascribed to the finite dimensions (diameter, 25 nm; height, 0.92 nm) of the clay platelets.12,13 The isomorphic substitution of some magnesium cations with lithium in the central sheet induces a partially negative charge. This charge is balanced by the presence of sodium ions in the interlayer space.12 Various ionic organic molecules can be easily intercalated in the interlayer of LAPONITE® through ion exchange. Recently, the increasing attentions have been focused on the IL-modified LAPONITE® because of the combination of the advantages of them, leading to the applications in catalysis, antibacterial, luminescence.14–16 For instance, Joshi et al.17 reported the coexistence of unique LAPONITE® colloidal gel–glass when LAPONITE® were dispersed in the ILs solution. Fraile et al.18 immobilized [Bmim][PF6] on the LAPONITE® surface and found that a solid phase was obtained at low coverage by solid NMR analysis; they speculated that the solid layer of [bmim][PF6] interacts with the surface through the protons in α position to the imidazolium ring. Nevertheless, the reported investigations only focused on the phase behavior of the LAPONITE® and the loaded ILs at room temperature. No systematic analyses have been performed on the cation–anion relative position and the molecular orientation of ILs incorporated into the interlayers of LAPONITE®, especially with the synergy between the temperature-dependent effects and the nanoconfinement.
In the present study, we mainly performed an in situ synchrotron radiation XAFS analysis with aid of TEM, DSC, IR and XRD methods to probe the cation–anion relative position and the molecular orientation of C6mimBr IL intercalated in LAPONITE®. We firstly investigated the crystallization and temperature-dependent structure deflection of C6mimBr ILs intercalated in LAPONITE® surface. Our results will provide a detailed theoretical basis for understanding the structural changes of ILs intercalated in LAPONITE® with the synergy between the temperature-dependent effects and the nanoconfinement.
Materials and methods
Materials
LAPONITE® (LAP, RD grade) was obtained from Southern Clay Products, Inc. The 1-butyl-3-methylimidazolium bromide (C6mimBr) used in our investigation was purchased from Sigma (purity 99%). The ILs were purified carefully in our laboratory by washing with water/CH2Cl2 to remove halogen impurities, followed by recrystallization, and the purity was confirmed by 1H NMR and HPLC. The properties of ILs are known to change with purity, particularly the water content. Therefore, the ILs were preheated at 70 °C in vacuum for 48 h before using.Methods
Up to 100 mg of C6mimBr was added to a suspension containing 600 mg of LAPONITE® RD and 100 mL of C2H5OH. This mixture was stirred at room temperature for 3 days. Afterward, the final product (C6mimBr/LAPONITE®) was generated by drying at 100 °C for 24 h under high vacuum to remove C2H5OH completely.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 658 eV) of the samples were recorded at room temperature in transmission mode by using ion chambers at the beam line BL14W1 of Shanghai Synchrotron Radiation Facility. The station was operated using a Si(III) double-crystal monochromator. During measurement, the synchrotron was operated at an energy of 3.5 GeV and a current of 230 mA. The photon energy was calibrated using the first inflection point of the Se K-edge in selenium metal foil. Data was analyzed using IFEFFIT.19 The k-space range was set from 1.2 Å−1 to 4.2 Å−1. The theoretical backscattering phase was calculated using the FEFF 8.2 code.20
658 eV) of the samples were recorded at room temperature in transmission mode by using ion chambers at the beam line BL14W1 of Shanghai Synchrotron Radiation Facility. The station was operated using a Si(III) double-crystal monochromator. During measurement, the synchrotron was operated at an energy of 3.5 GeV and a current of 230 mA. The photon energy was calibrated using the first inflection point of the Se K-edge in selenium metal foil. Data was analyzed using IFEFFIT.19 The k-space range was set from 1.2 Å−1 to 4.2 Å−1. The theoretical backscattering phase was calculated using the FEFF 8.2 code.20Results and discussion
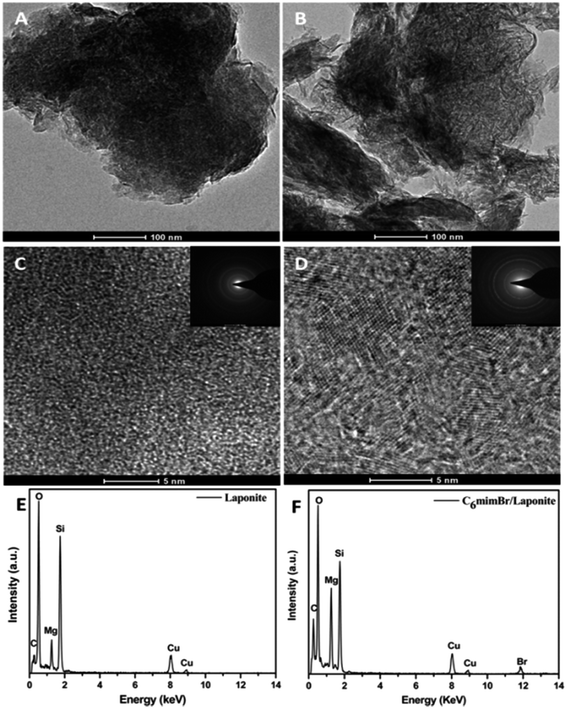
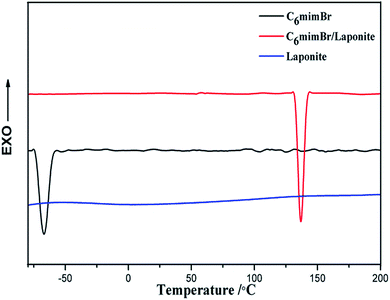
Fig. 1 shows TEM images, high resolution TEM (HRTEM) images and the corresponding energy-dispersive X-ray (EDX) spectra of the LAPONITE® and LAPONITE® intercalated with C6mimBr (C6mimBr/LAPONITE®). The EDX spectra of C6mimBr/LAPONITE® (Fig. 1F) confirmed the existence of Br atom, indicating the successful adsorption of C6mimBr ILs on the LAPONITE®. On the basis of the ion chromatography analysis, the amount of bromide bounded to LAPONITE® is approximately 0.45 wt%. Compared with HRTEM image of the LAPONITE® (Fig. 1C), some crystal planes can obviously be observed in HRTEM image of C6mimBr/LAPONITE® (Fig. 1D), demonstrating the formation of crystal for intercalated C6mimBr ILs. Moreover, selected area electron diffraction pattern (inset in Fig. 1D) clearly indicates the formation of C6mimBr crystals.To investigate the phase behavior change of C6mimBr ILs, differential scanning calorimetry (DSC) experiment was carried out. Fig. 2 displays the DSC heating curves for C6mimBr, C6mimBr/LAPONITE® and LAPONITE®. No fusion peaks for LAPONITE® can be observed at temperature below 200 °C, implying that the melting of LAPONITE® is above 200 °C. The melting point of pure C6mimBr was −66 °C, while one sharp fusion peak at 137 °C was clearly observed in the DSC curve of C6mimBr/LAPONITE®. The melting point of the C6mimBr IL on the LAPONITE® increased by ca. 200 °C compared with that of the neat IL, indicating the transition of C6mimBr from liquid to crystal. Obtaining stable IL crystals is very difficult or nearly impossible by simply cooling because solidification of IL by cooling often results in the glass formation.21 However, the TEM and DSC analyses indicated the formation of C6mimBr crystal by intercalating the C6mimBr ILs on the layer of LAPONITE®. In order to more clearly clarify the formation of C6mimBr crystal on LAPONITE®, XAFS analysis was further carried out.
Fig. 3 shows the X-ray absorption near edge structure (XANES) spectra and the corresponding Fourier transform (FT) R-space spectra for the C6mimBr and C6mimBr/LAPONITE® at ambient temperature. At the Br K-edge, the intensity and position changes of the white line peak at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 476 eV can be interpreted by variations in the electron density of the Br center atom. As shown in Fig. 3A, compared with pristine C6mimBr ILs, the intensity increase and the shift to higher energy side of the white line peak for the C6mimBr/LAPONITE® were attributed to the electron loss of the Br−. The electron loss of the Br− possibly resulted from the charge transfer from anion to cation in intercalated C6mimBr and from anion to LAPONITE®, implying the existence of IL–IL and IL–LAPONITE® interactions.
476 eV can be interpreted by variations in the electron density of the Br center atom. As shown in Fig. 3A, compared with pristine C6mimBr ILs, the intensity increase and the shift to higher energy side of the white line peak for the C6mimBr/LAPONITE® were attributed to the electron loss of the Br−. The electron loss of the Br− possibly resulted from the charge transfer from anion to cation in intercalated C6mimBr and from anion to LAPONITE®, implying the existence of IL–IL and IL–LAPONITE® interactions.
To obtain a clear local structure around the Br atoms, a series of FT R-space spectra for the C6mimBr and C6mimBr/LAPONITE® at ambient temperature are given in Fig. 3B. These spectra provide the definite distance between the anion and cation of C6mimBr. As shown in Fig. 3B, the distances between the Br− anions and [C6mim]+ cations in the C6mimBr and C6mimBr/LAPONITE® are 2.61 Å and 2.42 Å, respectively, implying that the distance between Br− and [C6mim]+ was significantly decreased for the IL after intercalating in the LAPONITE®. The shorter distance between anions and cations will lead to the enhancement of weak interactions (such as electrostatic forces and H-bonding in C6mimBr ILs). Notably, the new peak at 3.51 Å, ascribing to the O–H⋯Br bond, appeared in the spectrum for C6mimBr/LAPONITE®. This result indicates the formation of hydrogen bond between Br anion of the C6mimBr and O–H group of LAPONITE®, which is consistent with XANES analysis to confirm the existence of ILs–LAPONITE® interactions. This finding was further certified by FT-IR spectra of the LAPONITE®, C6mimBr, and C6mimBr/LAPONITE® and the results are shown in Fig. 4. As shown in Fig. 4, the FT-IR spectrum of the LAPONITE® shows a broad peak at 3445 cm−1 attributed to the Si–OH groups present on the edge of the clay.22 By contrast, vibration bands at 3705 and 3666 cm−1 were observed for the C6mimBr/LAPONITE®, indicating that the anion Br− strongly interacted with the hydroxyl groups of the outer surface of LAPONITE®. Similar result was investigated by Yang's group,23 they observed that the IR vibration bands at 3648 and 3572 cm−1 for BmimBF4 IL–SiO2 was indicative of the existence of the stronger interaction between the anion and the hydroxyl group of silanols. From the perspective of the formed hydrogen bonds, bromide ions locate on the outer edge of LAPONITE® and are not incorporated within the interlayer space when C6mimBr IL is intercalated in LAPONITE®. Similar results were reported by Bourgeat-Lami groups,24 concluded that monoalkoxy silanes form a flat layer on the edge of the clay by a condensation reaction with the clays edge silanol groups. Additionally, LAPONITE® presents a layered structure and exchangeable sodium cations in the interlayer space to compensate the excess layer charge. As a result, C6mim+ cations can intercalate in the interlayer nanospace by ion-exchange reaction. The major driving force for ion-exchange reaction should account for a coulombic stabilization by the neutralization of the charges on the LAPONITE®. This finding was further indicated by XRD pattern of the LAPONITE® and C6mimBr/LAPONITE® at ambient temperature and the results are shown in Fig. 5. As shown in Fig. 5, the diffraction peak at 2θ = 6.86° was attributed to the characteristic diffraction plane (001) of LAPONITE®. It is worth noting that the 2θ at 6.86° (d001 = 1.28 nm) in the LAPONITE® shifts to 6.32° (d001 = 1.39 nm) in the C6mimBr/LAPONITE®, indicating the intercalation of C6mim+ cations into the LAPONITE® interlayer. Moreover, we obtained an interlamellar space of 0.47 nm by subtracting the thickness of the clay layer (the thickness of the clay layer is approximately 0.92 nm (ref. 25)) in LAPONITE®/C6mimBr (d001 = 1.39 nm). The structure of C6mim+ was calculated by Gaussian-09 C1 package, as shown in Fig. S1 (ESI†), with the molecular dimensions of C6mim+ (10.8 × 4.2 × 3.6 in Å). This result suggests an inclination disposition of the long molecular axes of C6mim+ along the clay layers. The tilt angle (θ) of C6mim+ in the LAPONITE® interlayer can be calculated as follows:26
Δd = d001 − 0.92 = (ionic length)sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (1) |
Based on the above analysis, we draw a conclusion that the enhanced interactions in intercalated ILs, the hydrogen bonds formed between the Br anion of ILs and the OH group of LAPONITE®, as well as the ordered monolayer arrangement between intercalated cations and anions induced the formation of C6mimBr crystals intercalated in the LAPONITE®, which is in accordance with the appearance of new peak at 2θ = 8.84°, 21.18°, 25.80°, 26.78°, 28.90°, 39.37°, 42.76°, 50.82°, and 53.08°, belongs to the different diffraction planes of C6mimBr ILs crystal, in XRD pattern at ambient temperature (Fig. 5).
As the practical applications of ILs have considerable relevance with the temperature effect, the temperature-dependent microstructure changes of the intercalated ILs should be investigated to expand their applications. In our work, an in situ temperature XAFS experiment was performed to reveal the structural characteristics of the intercalated ILs at varied temperatures. Fig. 6 shows the XANES spectra and FT R-space spectra of C6mimBr/LAPONITE® at varied temperature, 25, 90, 120 and 160 °C, respectively. As shown in Fig. 6A, the slightly decreased intensity and the shift of white line peak to lower energy can be investigated with raising temperature from 25 °C to 120 °C, indicating the electron reception of the Br−. These results maybe contribute to the slight cleavage of hydrogen bond between Br anion of C6mimBr ILs and O–H groups of LAPONITE®. The electronegativity of Br atoms was stronger than that of the hydrogen atom in O–H group of the LAPONITE®, leading to a charge transfer from LAPONITE® to Br anion. However, the slight cleavage of hydrogen bond is not enough to change the structure of the intercalated ionic liquid. Therefore, with increasing temperature from 25 °C to 120 °C, the structure of intercalated ILs has no significantly change and maintains an ordered monolayer structure. However, a sudden decrease in intensity of the white line peak for the C6mimBr/LAPONITE® at 160 °C may be attributed to the complete cleavage of hydrogen bond.
To obtain the local structure around the Br atoms, a series of FT R-space spectra for the C6mimBr/LAPONITE® at varied temperatures are shown in Fig. 6B. As shown in Fig. 6B, the distance between the Br anion and [C6mim]+ cation in C6mimBr/LAPONITE® slightly increased with increasing the temperature from 25 °C to 120 °C. Singh et al.28 concluded that the mobility of ion confined in a slit-like graphitic nanopore increases with increasing temperature. Similarly, the increasing temperature promoted the movement of intercalated C6mim+ cations. Therefore, the slightly increase in the distance between cations and anions with increasing the temperature from 25 °C to 120 °C can be interpreted by the slight structure deflection of intercalated C6mim+ cations, in which C6mim+ cations maintain the ordered monolayer arrangement. This finding was further indicated by XRD patterns of the C6mimBr/LAPONITE® at varied temperatures and the results are shown in Fig. 7. It is revealed that some C6mimBr crystal diffraction peaks can be still observed at 120 °C, indicating the existence of ordered crystal structure. Moreover, the intensities of diffraction peaks at 26.78°, 42.76° slightly decreased and no new diffraction peak appeared with increasing the temperature from 25 °C to 120 °C, indicating that the structure of ILs crystal has no obvious change in the temperature range 25–120 °C. It is worth noting that the 2θ (d001) of LAPONITE® shifts to the lower value with increasing temperature, implying the expanding basal spacing (d001) of LAPONITE®. The increase in basal spacing of LAPONITE® indicated the structure deflection of the intercalated monolayer of C6mim+ cation. The XRD patterns of C6mimBr/LAPONITE® at 120 °C display a d001 basal spacing (2θ = 6.11°) of 1.44 nm. The corresponding tilt angle of C6mim+ was calculated to be 28.80° based on formula (1). In terms of the statistical average value, we speculated that the monolayer of C6mim+ inclined to the silicate layer with angle change from 25.80° to 28.80°, corresponding to a temperature change from 25 to 120 °C. This result is consistent with XAFS spectra with temperature from 25 °C to 120 °C. Additionally, the distance (2.68 Å) between cation and anion in intercalated C6mimBr ILs at 160 °C have clearly changed when compared with that of intercalated C6mimBr IL at 120 °C (2.47 Å), it is very close to that of pristine ILs (2.61 Å). These findings indicate that the structure of intercalated IL altered from ordered crystal structure to disordered amorphous structure with increasing the temperature from 120 to 160 °C and the intercalated IL was completely melted at 160 °C. These results are in well agreement with the disappearance of characteristic diffraction peak of C6mimBr ILs at 160 °C (Fig. 7). Therefore, at temperature lower than the melting point of the intercalated ILs, the intercalated C6mim+ cations tend to deflect and maintain the ordered monolayer structure with the rising temperature. The proposed structure change is depicted schematically in Scheme 1.
 | ||
| Fig. 7 XRD patterns of C6mimBr/LAPONITE® at varied temperature, 25 °C, 90 °C, 120 °C and 160 °C, respectively. | ||
 | ||
| Scheme 1 Schematic demonstration on the structure deflection of C6mimBr ionic liquid intercalated in LAPONITE® with the rising temperature. | ||
Conclusion
Crystallization and temperature-dependent structure deflection of C6mimBr ILs intercalated in LAPONITE® were investigated by in situ XAFS, with the aid of TEM, DSC, IR and XRD measurements. The TEM and DSC analyses verified the formation C6mimBr crystal. The XAFS spectra, with a combination of IR spectra and XRD pattern, indicated the formation an ordered monolayer structure with C6mim+ cation intercalated in the interlayer nanospace and Br located on the edge of the LAPONITE®. Moreover, the formation of C6mimBr crystals on the LAPONITE® may result from the following three factors: (1) the enhanced interactions in intercalated ILs, (2) the formation hydrogen bonds between the Br anion of the ILs and the OH group of the LAPONITE®, (3) the ordered monolayer arrangement between intercalated cations and anions. Furthermore, the XAFS spectra, with aid of XRD pattern at varied temperatures, demonstrated that the structure of intercalated C6mim+ cations tend to deflect and maintain the ordered monolayer arrangement with the rising temperature. The ordered crystal structure still exists at 120 °C and disappears only at a higher temperature. Our results will provide a detailed theoretical basis for understanding the temperature-dependent structural changes of the ILs intercalated in LAPONITE®, and should be helpful in expanding many ILs applications in which the confinement effects or solid–liquid interfaces are related.Conflict of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (11079007, 21306220, 21276257, 91534109). XAFS experiment was carried out at beamline BL14W1 SSRF (Shanghai Synchrotron Radiation Facility).References
- Y. Zhao and T. Bostrom, Application of Ionic Liquids in Solar Cells and Batteries: A Review, Curr. Org. Chem., 2015, 19(6), 556–566 CrossRef CAS.
- H. Lin, P. Bai and X. Guo, Ionic Liquids for SO2 Capture: Development and Progress, Asian J. Chem., 2014, 26(9), 2501–2506 CAS.
- J. D. Holbrey and K. R. Seddon, The phase behaviour of 1-alkyl-3-methylimidazolium tetrafluoroborates; ionic liquids and ionic liquid crystals, J. Chem. Soc., Dalton Trans., 1999, 13, 2133–2139 RSC.
- B. Xin, C. Jia and X. Li, Supported Ionic Liquids: Efficient and Reusable Green Media in Organic Catalytic Chemistry, Curr. Org. Chem., 2016, 20(5), 616–628 CrossRef CAS.
- M. Shirani, A. Semnani, S. Habibollahi, H. Haddadi and M. Narimani, Synthesis and application of magnetic NaY zeolite composite immobilized with ionic liquid for adsorption desulfurization of fuel using response surface methodology, J. Porous Mater., 2016, 23(3), 701–712 CrossRef CAS.
- M. Sha, G. Wu, Q. Dou, Z. Tang and H. Fang, Double-Layer Formation of BmimPF6 Ionic Liquid Triggered by Surface Negative Charge, Langmuir, 2010, 26(15), 12667–12672 CrossRef CAS PubMed.
- M. P. Singh, R. K. Singh and S. Chandra, Studies on Imidazolium-Based Ionic Liquids Having a Large Anion Confined in a Nanoporous Silica Gel Matrix, J. Phys. Chem. B, 2011, 115(23), 7505–7514 CrossRef CAS PubMed.
- Y.-L. Wang and A. Laaksonen, Interfacial structure and orientation of confined ionic liquids on charged quartz surfaces, Phys. Chem. Chem. Phys., 2014, 16(42), 23329–23339 RSC.
- H. Zhou, M. Rouha, G. Feng, S. S. Lee, H. Docherty, P. Fenter, P. T. Cummings, P. F. Fulvio, S. Dai, J. McDonough, V. Presser and Y. Gogotsi, Nanoscale Perturbations of Room Temperature Ionic Liquid Structure at Charged and Uncharged Interfaces, ACS Nano, 2012, 6(11), 9818–9827 CrossRef CAS PubMed.
- X. Gong, A. Kozbial, F. Rose and L. Li, Effect of π–π Plus Stacking on the Layering of Ionic Liquids Confined to an Amorphous Carbon Surface, ACS Appl. Mater. Interfaces, 2015, 7(13), 7078–7081 CAS.
- H. Z. Cummins, Liquid, glass, gel: the phases of colloidal LAPONITE®, J. Non-Cryst. Solids, 2007, 353(41–43), 3891–3905 CrossRef CAS.
- R. Sasai, T. Itoh, W. Ohmori, H. Itoh and M. Kusunoki, Preparation and Characterization of Rhodamine 6G/Alkyltrimethylammonium/LAPONITE® Hybrid Solid Materials with Higher Emission Quantum Yield, J. Phys. Chem. C, 2009, 113(1), 415–421 CAS.
- M. Jaber and J.-F. Lambert, A New Nanocomposite: L-DOPA/LAPONITE®, J. Phys. Chem. Lett., 2010, 1(1), 85–88 CrossRef CAS.
- A. V. Martinez, J. A. Mayoral and J. I. Garcia, Pd nanoparticles immobilized in bmimPF6 supported on LAPONITE® clay as highly recyclable catalysts for the Mizoroki–Heck reaction, Appl. Catal., A, 2014, 472, 21–28 CrossRef CAS.
- A. Sharma, P. Prakash, K. Rawat, P. R. Solanki and H. B. Bohidar, Antibacterial and Antifungal Activity of Biopolymers Modified with Ionic Liquid and LAPONITE®, Appl. Biochem. Biotechnol., 2015, 177(1), 267–277 CrossRef CAS PubMed.
- Y. Yao, Z. Li and H. Li, Modification of Eu3+-beta-diketonate complex-intercalated LAPONITE® with a terpyridine-functionalized ionic liquid, RSC Adv., 2015, 5(87), 70868–70873 RSC.
- N. Joshi, K. Rawat and H. B. Bohidar, Coexistence of Iso-Nonergodic LAPONITE® Gel and Glass in 1-Methyl-3-Octylimidazolium Chloride Ionic Liquid Solutions, J. Phys. Chem. B, 2014, 118(23), 6329–6338 CrossRef CAS PubMed.
- M. Rosa Castillo, J. M. Fraile and J. A. Mayoral, Structure and Dynamics of 1-Butyl-3-methylimidazolium Hexafluorophosphate Phases on Silica and LAPONITE® Clay: From Liquid to Solid Behavior, Langmuir, 2012, 28(31), 11364–11375 CrossRef PubMed.
- J. J. Rehr and R. C. Albers, Theoretical approaches to X-ray absorption fine structure, Rev. Mod. Phys., 2000, 72(3), 621–654 CrossRef CAS.
- T. Burchardt, V. Hansen and T. Valand, Microstructure and catalytic activity towards the hydrogen evolution reaction of electrodeposited NiPx alloys, Electrochim. Acta, 2001, 46(18), 2761–2766 CrossRef CAS.
- W. Xu, L. M. Wang, R. A. Nieman and C. A. Angell, Ionic liquids of chelated orthoborates as model ionic glassformers, J. Phys. Chem. B, 2003, 107(42), 11749–11756 CrossRef CAS.
- S. Salleres, F. L. Arbeloa, V. Martinez, T. Arbeloa and I. L. Arbeloa, Photophysics of Rhodamine 6G Laser Dye in Ordered Surfactant (C12TMA)/Clay (LAPONITE®) Hybrid Films, J. Phys. Chem. C, 2009, 113(3), 965–970 CAS.
- H. Yang, C. Yu, Q. Song, Y. Xia, F. Li, Z. Chen, X. Li, T. Yi and C. Huang, High-temperature and long-term stable solid-state electrolyte for dye-sensitized solar cells by self-assembly, Chem. Mater., 2006, 18(22), 5173–5177 CrossRef CAS.
- E. Bourgeat-Lami, N. N. Herrera, J. L. Putaux, S. Reculusa, A. Perro, S. Ravaine, C. Mingotaud and E. Duguet, Surface assisted nucleation and growth of polymer latexes on organically-modified inorganic particles, Macromol. Symp., 2005, 229, 32–46 CrossRef CAS.
- M.-A. Neouze, J. Le Bideau, P. Gaveau, S. Bellayer and A. Vioux, Ionogels, new materials arising from the confinement of ionic liquids within silica-derived networks, Chem. Mater., 2006, 18(17), 3931–3936 CrossRef CAS.
- T. Wan, H. Xu, Y. Yuan and W. He, Preparation and photochemical behavior of a cationic azobenzene dye-montmorillonite intercalation compound, J. Wuhan Univ. Technol., Mater. Sci. Ed., 2007, 22(3), 466–469 CrossRef CAS.
- M. Ogawa and K. Kuroda, Photofunctions of intercalation compounds, Chem. Rev., 1995, 95(2), 399–438 CrossRef CAS.
- R. Singh, J. Monk and F. R. Hung, Heterogeneity in the Dynamics of the Ionic Liquid BmimPF6 Confined in a Slit Nanopore, J. Phys. Chem. C, 2011, 115(33), 16544–16554 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: The structure of C6mim+ simulated by Gaussian-09 C1 package. See DOI: 10.1039/c6ra18618a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |