Metabolic engineering of TiO2 nanoparticles in Nitzschia palea to form diatom nanotubes: an ingredient for solar cells to produce electricity and biofuel†
Shristy Gautama,
Mrinal Kashyapa,
Shradhey Gupta b,
Vikas Kumarb,
Benoit Schoefs
b,
Vikas Kumarb,
Benoit Schoefs c,
Richard Gordon
c,
Richard Gordon de,
Clayton Jeffryesf,
Khashti Ballabh Joshi*b and
Vandana Vinayak*a
de,
Clayton Jeffryesf,
Khashti Ballabh Joshi*b and
Vandana Vinayak*a
aDiatom DBT Research Lab, School of Applied Sciences, Dr H.S. Gour Central University, Sagar, M.P, India. E-mail: kapilvinayak@gmail.com
bDepartment of Chemistry, Dr H.S. Gour Central University, Sagar, M.P, India. E-mail: kbjoshi77@gmail.com
cMicroMar, Mer Molécules Santé, IUML—FR 3473 CNRS, University of Le Mans, Faculté des Sciences et Techniques, Avenue Olivier Messiaen, 72085 Le Mans cedex 9, France
dGulf Specimen Aquarium & Marine Laboratory, Panacea, FL 32346, USA
eC.S. Mott Center for Human Growth and Development, Department of Obstetrics & Gynecology, Wayne State University, 275 E. Hancock, Detroit, MI 48201, USA
fDan F. Smith Department of Chemical Engineering, Lamar University, Beaumont, TX 77710, USA
First published on 23rd September 2016
Abstract
Diatoms are nature's nanobot because they can be described as cells in a glass house. Three-dimensional nanobio-engineered frustules of diatoms have vast applications in nanomaterials and bio-photonics. The amorphous silica of diatom frustules is not a good semiconductor, but when coated with titanium dioxide (TiO2), the semiconducting efficiency of the frustules becomes adequate for use in numerous device applications, including solar cells. The metabolic incorporation of titanium dioxide nanoparticles in the diatom frustules is primarily used for the construction of titanium nanotubes. In the present study, TiO2 was metabolically inserted by a two-stage cultivation process and its incorporation in the diatom frustules was studied by spectroscopic and atomic force microscopic methods. It was found that diatom frustules metabolically inserted with nanostructured TiO2 by a two-stage cultivation in f/2 medium could replace nanostructured surface doping with titanium in dye-sensitized solar cells for heat and electricity production. The DSSC made from the titanium-doped diatom frustule has a power efficiency almost double (9.45%) that of the simple DSSC (4.20%) without any diatom frustule metabolically inserted with TiO2. Alternatively, these nanoarray diatom containing tubes may also be used for the construction of novel dye-sensitized solar cells, which may help in oozing lipids from living diatom cells, such as in algal cells, for the generation of an electric current.
Diatoms are photosynthetic organisms found in fresh and marine waters.1,2 They are a rich source of HVMs (high value molecules) that have wide applications in health, chemical and pharmaceutical industries.3 Besides the HVMs, silica nanoparticles in diatoms have applications in nanobiotechnology, in drug delivery, nanobiosensors and in the control of particles in micro and nanofluidics.4 Diatoms also play a important role in climatic change and as biomarkers and in forensic science.5 The silica wall of diatom frustules can be doped with various nanoparticles to form biosensors and optical and electrical devices.4,6 They are also the earth's primary oil producers responsible for fixing 25% of CO2, and 30% of crude oil is believed to come from diatoms.7 They are indeed nanobiorefineries and produce about 10 times more oil than any other seed crop.8 The depletion of resources and the hike in oil prices have supported the continuous harvesting of bioproducts while maintaining cell viability, known as cell milking, as an optimal procedure of obtaining oil from these tiny living factories rather than extracting. The existing closed photobioreactors, which use long shelf-life batteries, not only kill the algae but are not cost effective procedures; therefore, researchers aim to optimize different techniques, such as milking via centrifugation and ultrasonication, as an alternative measures to harvest oil from diatoms without cell sacrificing. The construction of chemical solar cells from frustules of TiO2-doped diatoms for efficient dye-sensitized solar cells has already been proposed.9 Earlier reported work9–12 regarding the metabolic insertion of TiO2 has clearly shown that tailoring diatom frustules with nanocrystalline TiO2 serves as an effective material for the dye-sensitized solar cell, as nanoporous filters, and as photonic crystals.13 To add to this, we are proposing a chemical solar cell from living diatom frustules doped with nanocrystalline TiO2 to generate a pulse electric field that can help to ooze oil from the diatoms without killing them.3,14
The three-dimensional (3D) nanostructured, porous, silica exoskeleton of diatoms may enhance the uptake of sunlight and focus it on their chloroplasts,1–21 thus increasing oil production. The frustules serve as structures that couple incoming light into waveguides having distinct photonic band gaps and thus behave as living photonic crystals, a key factor required for increasing the efficiency of DSSCs.22 For building frustules, diatoms take up dissolved silicic acid from the growth medium into their cytoplasm via silica deposition vesicles (SDVs).23 Since the expression ‘Diatom Nanotechnology’ was coined by Richard Gordon in 1988 to designate this concept, it has attracted attention from many nanotechnologists worldwide.24 Indeed, frustules have been doped with various elements such as boron,25–27 germanium28 and titanium.29 Different methods are used for Ti doping,30 such as P4VP (poly-4-vinyl pyridine) or citric acid, to dope Ti on diatoms from the hydrolysis of titanium(IV) isopropoxide (TIP), while some others have used TiCl4 under controlled pH.31,32 Ti doping was achieved in the living diatom Thallassiosira weissflogii via multiple dosing of the titanium precursor titanium(IV) bis-(ammonium lactato)-dihydroxide (TiBALDH) to the cell cultures.12 Here, the Ti insertion within the frustule did not provoke modification of the nanopore architecture, except for a minor change in the rib structure.12
Metal-doped diatom frustules present interesting features, such as an enhancement of Ge photoluminescence33 and electron transfer in electrolysis (B-doped frustules used as the cathode).25,26 Ti has good semiconductor properties and is used for photovoltaics, rocking chair lithium batteries, dynamic random access memories (DRAM), optical sensors and dye-sensitized solar cells.25,26,30,34 In this manuscript, we demonstrate that metabolically incorporated Ti forms nanotubes (Fig. 1) within the pores of Nitzschia palea, conferring to the living diatoms the properties of a dye-sensitized solar cell that can be used to promote lipid oozing within an electric field. Ti was preferred over Ge because Ge29 but not Ti35 inhibits the diatom growth. Nevertheless, Ti deposition in mitochondria can happen and this can inhibit the electron transport and production of superoxide radicals.36 In addition, Ge-doped frustules lose their pore and intrapore structure as compared to Ti-doped frustules, while Ge-doped frustules cannot withstand the hydrodynamic forces and crumble, whereas Ti frustules do not.
The two-stage feeding of Si(OH)4 and Ti(OH)4 resulted in the formation of unique nanotubes, since Si(OH)4 is hydrolysed by the SDV and long chain polyamaines (LCPM) to form siloxane bonds (Si–O–Si).37,38 The resulting silicon nanospheres form nanostructured diatom frustules, and during co-feeding under experimental conditions, the Si uptake is dominant as compared to TiO2. As the Si pool exhausts in the final stages of frustules formation at the time of pore formation and as Ti has a slow rate of uptake and absorption, this therefore favors the nanoarray deposition of Ti on the Si frustules of the diatoms.9 This is probably the reason why Si(OH)4 and Ti(OH)4 display similar 3D structures. This, together with the finding that SDV proteins, such as silicateins, are involved in Ti mineralization,39–41 led us to hypothesize that Ti is transported with Si from the growth medium to the frustules, which represent there will be a much higher concentration of Ti in the vicinity of the pores when compared to away from the pores. The high-resolution AFM micrograph of a fresh sample of a diatom shows a beautiful and neat arrangement of pores over the surface of the diatoms. These microscopic images were taken in order to establish the intact structures of the diatoms before any kind of treatment. Two-dimensional (2D) and 3D micrographs of the intact diatoms pores clearly reveals the ordered arrangement of pores (Fig. S1, ESI†).
The diatom frustules improve the efficiency of DSSC through the greater surface area provided by the nanostructured pore pattern, which provides resonance in the visible spectral range, thus increasing the probability of an electron being excited by a photon.10 The architecture of the latest generation DSSC incorporates a nanostructured surface on which TiO2 is deposited in order to achieve the maximum surface area for the dye to absorb as much light as possible. Diatom frustules could replace this artificial nanostructured surface.32 Also, in the meantime, diatoms could be used to incorporate elements like Ti, widely used in the DSSC industry, owing to its biogenic, high surface area, nanostructure and semiconducting property. The high dielectric constant and periodic structure of the frustules was assumed to increase the light trapping efficiency, thus increasing the overall current efficiency of the device. The metabolic insertion of Ge in diatom9 frustules has shown photoluminescence (PL) and electroluminescence (EL) in the spectral range42 and has resulted in changes in the frustules' pore array, though the frustules shape did not change.9,35 On the other hand, the metabolic incorporation of TiO2 has resulted in the formation of semiconductor TiO2 in DSSCs and showed an increased solar energy efficiency as high as 5.32%.43
In addition, AFM observations revealed that Ti deposition also occurred in the pores of diatom frustules (marked with yellow and red arrows in Fig. 2). The concept behind the use of a diatom and its silica frustules as a solar cell was that the silicon, which has a band gap in the range of 1.1 eV, shows broad solar absorption and behaves as a good semiconductor suitable for the solar energy conversion process.44,45 The silica-coated diatom structure would be an excellent electrode material for solar energy conversion. To determine how Ti is structured within the pores, Ti-fed diatoms were further analysed by SEM. The samples were washed with Milli-Q water followed by methanol to remove extra contamination from/over the surfaces of the diatoms. Aliquots of diatom samples (5–10 μL, ESI†) were deposited over the surface of a 5 mm diameter glass mirror disc followed by gold sputter coating. Interestingly, we observed that all the pores of the diatom surface were filled. We observed the uniform deposition of Ti into the pores of diatoms. The diatom pores were filled with Ti, and no inner and outer deposition (Fig. 3) was seen during the SEM investigations, and the observation also well corresponded with the results from the AFM study.
Transmission electron microscopy of Ti-doped diatom nanotubes further confirmed its deposition on the 11th day of Ti insertion, as shown in Fig. 4. In the absence of Ti, the frustules' pores appear as bright spots, whereas in the Ti-fed samples these pores are filled with nanostructured material (marked by red arrows in Fig. 4B and C). Furthermore, the high resolution the TEM micrograph distinctly shows the lattice fringes where d, the distance between two lattice fringes, is ∼2.9 Å, which is typically observed for the crystallinity (Fig. 4D) of Ti anatase. This experiment suggests that the controlled deposition of Ti over the diatoms, i.e., the diatom's pores, and the crystalline nature of these nanoparticles can lead to useful nanodevices, which could show their potential application in biofuels generation and in other nanotechnological fields. Another important feature of the Ti metabolic insertion was that the addition of titanium had no effect on the overall growth of the diatom cells in the suspension media. Therefore, the lower or non-toxic concentration of Ti nanoparticles is also useful for developing bionanoprobes and sensors. The excellent photodynamic properties of Ti nanoparticles can be useful to modulate the diatom's material properties and hence can enable it to work as a good semiconductor for green energy sources.
The molecular recognition and specific interactions between the two or more systems or molecules can be very useful and is a keystone for the development of bionanotechnology. Bioactive molecules, such as proteins and polypeptides, can show an interaction with several kinds of metal ions. Hence, this interesting area of research has led to the development of methodologies to identify these biomolecular interactions and to further their application. Since the diatom cell wall contains several proteins, it is expected that these proteins will have aromatic amino acids, such as tryptophan, tyrosine and phenylalanine. Among these three amino acids, other than tyrosine-rich proteins, tryptophan-rich proteins are more convenient to study owing to their unique photophysical and photochemical properties. Therefore tryptophan-containing conjugates are primarily used by researchers as efficient probes to unravel biotechnological problems. The presence of tryptophan in any system is vitally useful and helps us to unravel the interactions of metal ions, such as Ti ions with diatoms, by spectroscopic tools. To confirm the presence of tryptophan/tyrosine residue in the diatom, we recorded the UV-Vis spectra of pure diatoms and a diatom in the presence of Ti(IV) ions, in water. Interestingly, we found that the absorbance at 280 nm, which perhaps confirms that the tryptophan/tyrosine residues are extruded from the surface of diatoms or are present near the surface of the diatoms (Fig. S6†). The UV-Vis study clearly revealed that the diatom surface is abundant with tryptophan/tyrosine residues. These tryptophan/tyrosine residues are the most well-studied fluorophores,46–48 and therefore, it is possible to study the molecular interactions of Ti ions with the diatom cell wall with the help of fluorescence methods. The presence of tryptophan on the diatom surface can work as a fluorescence probe and its fluorescence property could facilitate gathering the spectral evidence of non-covalent molecular interactions by fluorescence titration measurements. We experimentally observed the change in fluorescence intensity upon the gradual addition of Ti nanoparticles in a solution of diatoms, comprising both pure and denatured cultured diatom cells (Fig. 5).
 | ||
| Fig. 5 Spectra showing the fluorescence quenching of tryptophan residues of (a) pure diatoms and (b) denatured diatoms upon the incremental addition of Ti ions. | ||
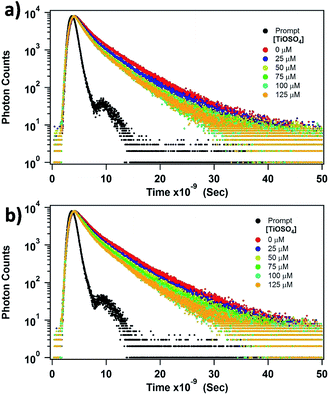
To estimate the quenching constant of the bimolecular interactions, we acquired the lifetime of tryptophan present on the diatom surface, of the pure and denatured diatoms, as shown in Fig. 6. The experimental data decay profiles were fitted by a bi-exponential function. Quenching data were analysed according to the Stern–Volmer plot by plotting τo/τ vs. [TiOSO4], where τo and τ are the unquenched and quenched fluorescence lifetime and Ti(IV) is the concentration of the quencher. The Stern–Volmer plots in both cases (in the pure and denatured cases diatoms) are shown in Fig. 7. Surprisingly, the Stern–Volmer plot behaves absolutely non-linear in the pure diatom case; however, the denatured diatom reveals to be almost linear. The perceivable explanation for the non-linear variation in the pure diatom is that a few tryptophan residues are buried in the diatom interior and are relatively inaccessible to TiOSO4, while the other tryptophan residue is on the surface of the diatom and is more accessible.49,50 This could lead to a non-linear variation in the Stern–Volmer plot. Also, when executing the quenching experiments, it is important to consider whether the quencher has an adverse effect on the diatom's surface. Quenchers like Ti(IV) ions are evident here to bind to the diatom and induce conformational changes in proteins present at the diatom's surface. In order to calculate the quenching constant in the pure diatom case, we used the following equation to fit the quenching data (τo/τ vs. Ti(IV)):49,50
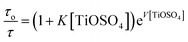 | (1) |
For a homogeneously emitting system, the quenching process can be described by the dynamic and static quenching constants, K and V.49 The dynamic and static quenching constants describe the process of quenching, where dynamic or collision quenching is normally observed with the collision of an excited state fluorophore and another molecule in solution; on the other hand, in static quenching, fluorophores form a reversible complex with the quencher molecule in the ground state, which does not rely on diffusion or molecular collisions.50 In our case, both these quenching processes occur simultaneously in the pure diatom case. It is our belief that the observed quenching in the present system is a result of either a photo-induced electron transfer (PET) process or a Förster resonance energy transfer (FRET) process, or perhaps a combination of both processes between the Ti(IV) ions and cell wall proteins of the diatoms, which clearly reveals that Ti ions interact with the diatom cell. The observed values of the dynamic and static quenching constants for pure diatoms are 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634 M−1 and 5230.5 M−1, respectively. Aside from this, in the case of the denatured diatom, where all the tryptophan is accessible, this exhibits an almost linear variation (Fig. 7). Hence, the quenching data can be well fitted by using the Stern–Volmer equation as follows:50
634 M−1 and 5230.5 M−1, respectively. Aside from this, in the case of the denatured diatom, where all the tryptophan is accessible, this exhibits an almost linear variation (Fig. 7). Hence, the quenching data can be well fitted by using the Stern–Volmer equation as follows:50
 | (2) |
In the present case, the dynamic quenching constant was obtained as 4211.6 M−1. This suggests that the observed quenching involved the collision of an excited state tryptophan with Ti(IV) ions. From the fluorescence study, it is clearly evident that the pure diatom exhibits both dynamic and static quenching; however, only dynamic quenching is apparent in the case of denatured diatoms. This means that tryptophan residues form a reversible complex with the Ti ions in the ground state with additional collision quenching by free Ti ions in solution. The spectroscopic study supports the imaging data where Ti ions are found to be deposited physically and chemically on the diatom surface.
Preliminary microscopic (AFM) and spectroscopic observations clearly reveal that Ti interacts with the diatom's cell wall and bind with the surface protein. Therefore, the deposition of Ti is on the frustule pores and our observations reveal that the preferential deposition sites are the pores of N. palea because near the pores of the diatom the associated proteins are more accessible. We also demonstrated the time-dependent deposition of Ti and its effect on the diatom's growth. The AFM image of the diatoms treated with Ti on the 10th day (Fig. 8A and B) depicted that not all the pores of the diatoms were filled and in some cases the pores are progressively filled with Ti nanoparticles. We can consider this phase as a nucleation phase for the next step, in which the energy of the system is at a maximum. Upon prolonged incubation of Ti upto the 25th day after feeding on the 10th day, the maximum numbers of pores are filled with Ti nanoparticles (Fig. 8C and D). This observation confirms that, for adequate deposition, we need to incubate the diatoms with a Ti solution for a minimum of 18 days after feeding on the 10th day and thereafter the diatoms should be subcultured for a viability check. In case we incubate it for more than 18 days, the system is at minimum energy and then reaches the saturated state, and in this time, not only are the pores of the diatoms filled, but an over-deposition of Ti around the diatom pores was also observed (Fig. 8E and F).
The titanium was preferentially deposited as a nanophase lining the frustules of each diatom, which is a prerequisite for the formation of a DSSC. At stage-I, the diatom cells were in a Si-starved condition and on the 10th day of growth, the silicon delivery rate was fixed, whereas the Ti delivery rate was varied by changing the Ti concentration (1.25 mM to 0.125 mM) in the feed solution as per the protocol followed by Jeffryes et al.35 The AFM study revealed the incorporation of titanium into the pores of N. palea as elevated ridges as compared to the hollow wells in the control N. palea without Ti metabolic insertion (Fig. S1A, ESI†).
To add additional novelty to our concept, we combined Reep & Green's14 procedure for extracting lipids from algae without cell sacrifice. A repetitive pulse from an electric field with a frequency of 2 Hz and voltage strengths of 3 and 6 kV cm−1 induced the extraction of proteins from Chlorella vulgaris Beyerinck (Trebouxiophyceae) and Haematococcus pluvialis Flotow (Chlorophyceae), which have hard cell walls.46 An incubation time was further required for the oil to get collected and for the cells to heal their fractured cell wall.3,35,52 The prototype model showed that a small DC discharge of 12.5 V with a minimal current for a time of 3 min resulted in the release of an oily substance on the surface of water. The microscopic inspection of the water-containing algal biomass showed minor cell wall fracturing along with the oil release. We checked the revival rate of diatom cells after each treatment and found good to excellent results because all the cells had a good amount of chloroplast, which is essential for photosynthesis (Fig. 9).
The algal biomass was preserved and inspected after several days. By microscopy, the sample was seen to show no signs of cell damage and the individual cells remained healthy and normal.
Table 1 shows that after the two-stage cultivation of feeding with Ti 0.125 mM to 1.25 mM (400 μL h−1) for 10 h and silica 200 mM (400 μL h−1) on the 10th day of inoculation in 200 mL of f/2 media containing N. palea cells, there was no effect on the growth of the cells (Table 1) and the cells grew exponentially from 500 ± 50 cells on the 10th day, to 595 ± 50 cells on the 11th day and 723 ± 50 cells per mL on the 12th day per 10 μL of 200 mL of batch culture. The lipid content estimated from the diatom53 Ti nanotubes also showed a substantial lipid percentage per 1 mL of cell volume, which was 0.2% on the 10th day, 0.4% on the 11th day and 0.6% on the 12th day increasing to 1.0% on the 18th day. On the 21st day, the cells were checked for the revival rate by taking inoculums and transferring them to fresh media. Cell growth was resumed, as seen in Fig. 9.
| Sl. no. | Day (s) | Average count/mL | Obtained lipid content percentage |
|---|---|---|---|
| 1. | Control | 3000 | 0.09% |
| 2. | 10th day | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.2% |
| 3. | 11th day | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
0.4% |
| 4. | 12th day | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
0.6% |
| 5. | 14th day | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
0.7% |
| 6. | 16th day | 104![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
0.8% |
| 7. | 18th day | 118![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.0% |
Next, to check the application of Ti nanoparticles-doped diatoms as a dye-sensitized solar cell (DSSC), we performed the following experiments. The two DSSC cells were prepared in quintuplets and for them, one FTO plate, which acted as an anode, was coated with TiO2.56 Fig. 3A shows the dye-sensitized solar cell with TiO2 as a control and Fig. 3B the diatom DSSC with diatom frustules doped with TiO2 by a two-stage cultivation.9 The average current produced in the control was 57.8 ± 1.08 μA, which was 2.5 times less than that produced by the frustules Ti-doped DSSC (128.8 ± 3.26 μA). The power efficiency of the control DSSC was 4.20%, while that of the test DSSC with the TiO2-doped diatom was 9.45% (Fig. 10). The latest generation DSSC in the present study was applied on a nanostructured surface on which TiO2 was deposited in order to achieve the maximum surface area for the dye to absorb as much light as possible. Diatom frustules could replace this artificial nanostructured surface.57 Also, diatom frustules could be optimized by tailoring the pore size and structure or by using the natural biological machinery of diatoms to incorporate elements like TiO2, which is widely used in the DSSC industry, into biogenic, high surface area, nanostructured and semiconducting frustules.
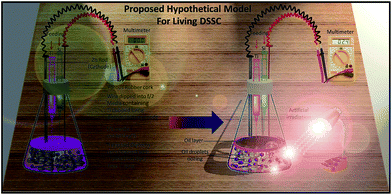
The amorphous silica of the diatom frustules is not a good semiconductor, but when coated with titania the semiconducting efficiency of the frustules become adequate for use in numerous device applications, including solar cells. Here, we also propose a biogenic dye-sensitized solar cell model (Fig. 11) based on living diatoms to generate an electric pulse of sufficient intensity to fracture the diatom frustules, which facilitates the concurrent oozing of oil droplets into the culture medium without cell sacrifice. This process primarily involved the construction of titanium nanotubes in the diatom frustules, where TiO2 was metabolically inserted by a two-stage cultivation process. The feeding of silica in two cultivation stages in f/2 medium supplemented with Lugol's iodine as an electrolyte and ruthenium dye as a photoactive dye generated electric pulses that fractured the frustule, causing lipid drops to ooze from the cells to the culture medium on the 13th day of inoculation. The diatoms healed their frustules when fed with nutrient media and revived after the 26th day of inoculation. This process leads to the conversion of living diatoms into an efficient dye-sensitized solar cell, which can further extract more oil than diatoms do naturally and represents a novel method of building an economical living solar cell that simultaneously produces biofuels. A repetitive pulse from an electric field with a frequency of 2 Hz and voltage strengths of 3 and 6 kV cm−1 induced the extraction of proteins from Chlorella vulgaris Beyerinck (Trebouxiophyceae) and Haematococcus pluvialis Flotow (Chlorophyceae), which have hard cell walls.51 A incubation time was further required for the oil to get collected and for cells to heal their fractured cell wall.3,35,52 The prototype model showed that a small DC discharge of 12.5 V with a minimal current for a time of 3 min resulted in the release of an oily substance on the surface of water. The microscopic inspection of the water-containing algal biomass showed minor cell wall fracturing along with the oil release. The algal biomass was preserved and inspected after several days. By microscopy, the sample showed no signs of cell damage and the individual cells remained healthy and normal.14
Conclusions
In this paper, we proposed combining the idea of the metabolite insertion of nanostructured TiO2 into patterned diatom biosilica (Fig. 1) and converting it into a dye-sensitized solar cell.56 Thus, in this study, we propose to combine the idea of metabolite insertion of nanostructured TiO2 into the patterned diatom biosilica (Fig. 1) and converting it into dye sensitized solar cell. Once the living diatoms are converted into living DSSCs, the electric current generated fractures the cell wall of the diatom enough to extract the lipids from the algal cells without cell sacrifice. The biofabrication of titanium in Nitzschia palea was done for the first time for the living diatom frustules. Earlier studies used Pinnularia sp., Coscinodiscus wailesii and Thalassiosira weissflogii, respectively, as the candidates for diatom-nanostructured material construction. N. palea showed a beautiful incorporation of titanium in the pores and a nanoarray of titanium deposition when no other parameter was changed serves as a useful application for the construction of living diatom solar cells for electric current, heat, and also for biofuel generation. Reep and Green used an electric current to fracture the cell wall of microalgae without actually sacrificing them. This way we proposed that a living nanoarray of titanium nanoparticles in diatom frustules can be of use in unique applications in the construction of living dye-sensitized solar cells (DSSCs), not only for heat and electricity but also to utilize that heat/electricity to fracture the cell wall of the diatom frustules to ooze oil from them and to let them revive when fed suitable nutrient media.Experimental section
The preparation of Ti 5 mM and Si 200 mM was done as per the reported protocol,35 and standards of Ti were prepared using UV-Vis spectrophotometry (Labindia analytical, UV 3000+) and that of silica to calculate the concentrations of Ti and Si in the media. The concentration of Ti and Si was standardized for feeding 200 mL of diatom cell culture. It was found that a Ti concentration ranging from 0.125 mM to 1.25 mM and a fixed 200 mM concentration of Si at the rate of 400 μL h−1 for 10 h can help in maintaining the total pH of the media at 7.5.Bioreactor cultivation for the metabolic insertion of titanium into frustules biosilica
A two-stage photobioreactor cultivation process proposed by Jeffryes et al.35 was constructed with a little modification, whereby instead of autofitted vents, syringes were used. The experiment was done in a flask (250 mL) with the feeding of titanium and silica done by syringes manually from the sides of a sterile cotton plug. A total of 3000 cells per mL were feeded in the modified f/2 culture medium.54 In stage-I of cultivation the diatom cell suspension of Nitzschia palea (Kutzing) W. Smith was grown on modified f/2 media54 at 0.50 mM Sodium metasilicate (Na2SiO3·9H2O) concentration. In stage-II of the cultivation, 200 mM sodium metasilicate and soluble TiOSO4 400 μL h−1 (concentration: 0.1.25 mM to 1.25 mM) for 10 h was fed to the diatom suspension medium simultaneously using an autopipette over a period of 10 h (Table 1). The metabolic insertion of Ti in diatom N. palea was studied by AFM.Preparation of DSSC and DSSC with diatom frustule doped with TiO2
FTO plates L 50 mm × W 50 mm × D 2.2 mm, resistivity – 7 ohms sq.−1, film thickness 1400–1500 Å were purchased from Techinstro, Nagpur, India. The concentration and amount of all the ingredients used in the DSSC was fixed for both the control and the test cells. The DSSC with only TiO2 was labelled as the control and the other DSSC with the TiO2 coating with an additional coating of diatom frustules doped with TiO2 by a two-stage cultivation method as proposed by Jeffryes9 was labelled as the test. The electrodes of the control and test were baked at 400 °C for 1 h. The other electrode of the DSSC was prepared by coating the carbon soot from flame onto the conductive side of an FTO plate (ESI Fig. S7†). The DSSCs for the control and test were prepared by the reported protocol of O'regan and Grätzel56 using di-tetrabutylammonium cis-bis(isothiocyanato)bis(2,2′-bipyridyl-4,4′-dicarboxylato)ruthenium(II) (3 × 10−4 M) purchased from Sigma Aldrich (Fig. 12) and a redox electrolyte Lugol's iodine 50 mM. The current in microamperes was measured for the control and tests (n = 5 each) upon irradiating the prepared DSSC cell to 10 V artificial light directly. | ||
| Fig. 12 Structure of di-tetrabutylammonium cis-bis(isothiocyanato)bis(2,2′-bipyridyl-4,4′-dicarboxylato)ruthenium(II). | ||
Atomic force microscopy
The diatom samples (1 μL) were placed on the HOPG surface (highly ordered pyrolytic graphite) and were imaged with an atomic force microscope (Sophisticated Instrument Center, Dr Harisingh Gour Central University, Sagar), (INNOVA, ICON analytical equipment, Bruker), operating under the acoustic AC mode (AAC or tapping mode), with the aid of a cantilever (NSC 12(c) from MikroMasch, silicon nitride tip) using a small area scanner and were scanned in the maximum range of 5 μm, followed by image processing using NanoDrive™ version 8 software. The force constant was 2.0 N m−1, while the resonant frequency was 284.60 kHz. The images were taken in air at room temperature, with a scan speed of 1.8–2.2 lines per s. The data analysis was done using Nanoscope Analysis Software. Diatom solutions were taken from the stock solution in an small eppendorf tube and centrifuged for 5 min, and after removing the excess supernatant, a 1 μL aliquot of the aqueous layer was spread on a mica sheet and fixed by using methanol.Spectroscopic methods
Fluorescence spectra were recorded on an RF-5301PC spectrofluorophotometer (Shimadzu) with a 10 mm quartz cell at room temperature. The fluorescence lifetime was measured by single photon counting method using a commercial setup from Jobin-Yvon. For the lifetime measurement, samples were excited at 287 nm using a nano-LED and the emitted photons were detected at 350 nm wavelength.Cell count
The diatom cells were counted using a Neubauer chamber.55Estimation of lipid content
The lipid content was estimated by Abubakar et al.'s53 method.Acknowledgements
MK thanks UGC India for pre-doctoral research fellowship (JRF), VK thanks NFOBC-UGC-India for pre-doctoral research fellowship, SG thanks CSIR-India for postdoctoral (RA) research assistance. SG, KBJ and VV thank CSIR-India and DBT-India respectively for financial assistance. Authors thank SIC-DHSGU Sagar-India and Department of chemistry, IIT Kanpur for AFM/SEM facility and lifetime measurements respectively.Notes and references
- T. Hisatomi, J. Kubota and K. Domen, Chem. Soc. Rev., 2014, 43(22), 7520–7535 RSC
.
- T. L. N. N. Deroche, A. Caruso, T. T. Le, T. V. Bui, B. Schoefs, G. Tremblin and A. M. Manceau, Sci. World J., 2012, 1–15 CrossRef PubMed
.
- V. Vinayak, K. M. Manoylov, H. Gateau, V. Blanckaert, J. Hérault, G. Pencréac'h, J. Marchand, R. Gordon and B. Schoefs, Mar. Drugs, 2015, 13(5), 2629–2665 CrossRef CAS PubMed
.
- J. E. N. Dolatabadi and M. de la Guardia, Trends Anal. Chem., 2011, 30(9), 1538–1548 CrossRef CAS
.
- I. Atazadeh, M. Sharifi and M. Kelly, Hydrobiologia, 2007, 589(1), 165–173 CrossRef CAS
.
- D. Losic, J. G. Mitchell and N. H. Voelcker, Adv. Mater., 2009, 21(29), 2947–2958 CrossRef CAS
.
- T. Ramachandra, D. M. Mahapatra and R. Gordon, Ind. Eng. Chem. Res., 2009, 48(19), 8769–8788 CrossRef CAS
.
- T. Whittington, “Biodiesel production and use by farmers: is it worth considering”, Department of Agriculture and Food, Western Australia, 2006 Search PubMed
.
- C. Jeffryes, T. Gutu, J. Jiao and G. L. Rorrer, Mater. Sci. Eng., C, 2008, 28(1), 107–118 CrossRef CAS
.
- C. Jeffryes, J. Campbell, H. Li, J. Jiao and G. Rorrer, Energy Environ. Sci., 2011, 4(10), 3930–3941 CAS
.
- M. S. Chauton, L. M. B. Skolem, L. M. Olsen, P. E. Vullum, J. Walmsley and O. Vadstein, J. Appl. Phycol., 2015, 27(2), 777–786 CrossRef PubMed
.
- Y. Lang, F. del Monte, B. J. Rodriguez, P. Dockery, D. P. Finn and A. Pandit, Sci. Rep., 2013, 3, 1–11 Search PubMed
.
- L. M. B. Skolem, Biosynthesis and characterization of Ti-doped silica-based Nanostructures formed by the Diatoms Pinnularia sp. and Coscinodiscus wailesii, Master’s thesis, Norwegian University of Science and Technology, 2011.
- P. Reep and M. P. Green, Procedure for extracting of lipids from algae without cell sacrifice, US Pat., Application No. 13/207, 298, 2012
.
- L. De Stefano, I. Rea, I. Rendina, M. De Stefano and L. Moretti, Opt. Express, 2007, 15(26), 18082–18088 CrossRef PubMed
.
- C. E. Hamm, R. Merkel, O. Springer, P. Jurkojc, C. Maier, K. Prechtel and V. Smetacek, Nature, 2003, 421(6925), 841–843 CrossRef CAS PubMed
.
- M. Sumper and E. Brunner, Adv. Funct. Mater., 2006, 16(1), 17–26 CrossRef CAS
.
- N. Kröger and N. Poulsen, Annu. Rev. Genet., 2008, 42, 83–107 CrossRef PubMed
.
- M. Hildebrand, Prog. Org. Coat., 2003, 47(3), 256–266 CrossRef CAS
.
- G. Di Caprio, M. A. Ferrara, L. Miccio, F. Merola, P. Memmolo, P. Ferraro and G. Coppola, J. Biophotonics, 2014, 7(5), 341–350 CrossRef CAS PubMed
.
- K. Roháček, M. Bertrand, B. Moreau, B. Jacquette, C. Caplat, A. M. Manceau and B. Schoefs, Philos. Trans. R. Soc., B, 2014, 369(1640), 1–8 CrossRef PubMed
.
- T. Fuhrmann, S. Landwehr, M. E. R. Kucki and M. Sumper, Appl. Phys. B: Lasers Opt., 2004, 78(3–4), 257–260 CrossRef CAS
.
- V. V. Annenkov, T. N. Basharina, E. N. Danilovtseva and M. A. Grachev, Protoplasma, 2013, 250(5), 1147–1155 CrossRef CAS PubMed
.
- R. Gordon, D. Losic, M. A. Tiffany, S. S. Nagy and F. A. S. Sterrenburg, Trends Biotechnol., 2009, 27(2), 116–127 CrossRef CAS PubMed
.
- Y. Zhang, Y. Wu, M. Chen and L. Wu, Colloids Surf., A, 2010, 353(2), 216–225 CrossRef CAS
.
- J. DeLoach, G. Scarel and C. Aita, J. Appl. Phys., 1999, 85(4), 2377–2384 CrossRef CAS
.
- S. Chandrasekaran, T. J. Macdonald, A. R. Gerson, T. Nann and N. H. Voelcker, ACS Appl. Mater. Interfaces, 2015, 7(31), 17381–17387 CAS
.
- D. Zhang, Y. Wang, J. Pan and J. Cai, J. Mater. Sci., 2010, 45(21), 5736–5741 CrossRef CAS
.
- D. Losic, Y. Yu, M. S. Aw, S. Simovic, B. Thierry and J. Addai-Mensah, Chem. Commun., 2010, 46(34), 6323–6325 RSC
.
- J. Toster, C. Harnagea, K. S. Iyer, F. Roseib and C. L. Raston, CrystEngComm, 2012, 14(10), 3446–3450 RSC
.
- P. Wilhelm and D. Stephan, J. Colloid Interface Sci., 2006, 293(1), 88–92 CrossRef CAS PubMed
.
- H. H. Choi, J. Park and R. Singh, Appl. Surf. Sci., 2005, 240(1), 7–12 CrossRef CAS
.
- P. Wilhelm and D. Stephan, J. Photochem. Photobiol., A, 2007, 185, 19–25 CrossRef CAS
.
- R. Hengerer, B. Bolliger, M. Erbudak and M. Grätzel, Surf. Sci., 2000, 460(1), 162–169 CrossRef CAS
.
- C. Jeffryes, T. Gutu, J. Jiao and G. L. Rorrer, ACS Nano, 2008, 2(10), 2103–2112 CrossRef CAS PubMed
.
- N. Li, C. Sioutas, A. Cho, D. Schmitz, C. Misra, J. Sempf, M. Wang, T. Oberley, J. Froines and A. Nel, Environ. Health Perspect., 2003, 111(4), 455–460 CrossRef CAS PubMed
.
- N. Kröger, R. Deutzmann and M. Sumper, Science, 1999, 286, 1129–1132 CrossRef
.
- N. Kröger, S. Lorenz, E. Brunner and M. Sumper, Science, 2002, 298, 584–586 CrossRef PubMed
.
- J. L. Sumerel, W. Yang, D. Kisailus, J. C. Weaver, J. H. Choi and D. E. Morse, Chem. Mater., 2003, 15(25), 4804–4809 CrossRef CAS
.
- K. E. Cole, A. N. Ortiz, M. A. Schoonen and A. M. Valentine, Chem. Mater., 2006, 18(19), 4592–4599 CrossRef CAS
.
- N. Kröger, M. B. Dickerson, G. Ahmad, Y. Cai, M. S. Haluska, K. H. Sandhage, N. Poulsen and V. C. Sheppard, Angew. Chem., 2006, 118(43), 7397–7401 CrossRef
.
- C. Jeffryes, R. Solanki, Y. Rangineni, W. Wang, C. Chang and G. L. Rorrer, Adv. Mater., 2008, 20(13), 2633–2637 CrossRef CAS
.
- J. Lin, J. Chen and X. Chen, Nanoscale Res. Lett., 2011, 6(1), 1–5 Search PubMed
.
- A. Goetzberger and C. Hebling, Sol. Energy Mater. Sol. Cells, 2000, 62(1), 1–19 CrossRef CAS
.
- A. Goetzberger, C. Hebling and H. W. Schock, Mater. Sci. Eng., R, 2003, 40(1), 1–46 CrossRef
.
- N. K. Mishra, K. B. Joshi and S. Verma, J. Colloid Interface Sci., 2015, 455, 145–153 CrossRef CAS PubMed
.
- N. K. Mishra, V. Kumar and K. B. Joshi, RSC Adv., 2015, 5, 64387–64394 RSC
.
- N. K. Mishra, V. Kumar and K. B. Joshi, Nanoscale, 2015, 7, 20238–20248 RSC
.
- M. Eftink and L. Selvidge, Biochemistry, 1982, 21(1), 117–125 CrossRef CAS PubMed
.
- J. Lakowicz, “Principles of Fluorescence Spectroscopy”, Springer, New York, 2006 Search PubMed
.
- M. Coustets, V. J. Durigneux, J. Hérault, B. Schoefs, V. Blanckaert, J. P. Garnier and J. Teissié, Bioelectrochemistry, 2015, 103, 74–81 CrossRef CAS PubMed
.
- M. P. Rols and J. Teissie, Eur. J. Biochem., 1989, 179(1), 109–115 CrossRef CAS PubMed
.
- L. Abubakar and A. Mutie, J. Appl. Phytotechnol. Environ. Sanit., 2012, 1(4), 147–153 CAS
.
- V. Vinayak, R. Gordon, S. Gautam and A. Rai, Adv. Sci. Lett., 2014, 1256–1267 CrossRef
.
- I. Moreno-Garrido, M. Hampel, L. Lubián and J. Blasco, Fresenius' J. Anal. Chem., 2001, 371(4), 474–478 CrossRef CAS PubMed
.
- B. O'regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef
.
- H.-H. Choi, J. Park and R. Singh, Appl. Surf. Sci., 2005, 240, 7–12 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra18487a |
| This journal is © The Royal Society of Chemistry 2016 |