Microfiltration process for surface water treatment: irreversible fouling identification and chemical cleaning†
Junxia Liu *ab,
Bingzhi Dong*b,
Bangqing Caoc,
Dongsheng Zhaob and
Zhihong Wanga
*ab,
Bingzhi Dong*b,
Bangqing Caoc,
Dongsheng Zhaob and
Zhihong Wanga
aFaculty of Civil and Transportation Engineering, Guangdong University of Technology, Guangzhou 510006, China. E-mail: whjunxia@163.com; Fax: +86 20 39322515; Tel: +86 20 39322515
bState Key Laboratory of Pollution Control and Resource Reuse, School of Environmental Science and Engineering, Tongji University, Shanghai 200092, China. E-mail: dbz77@tongji.edu.cn
cAcademy of Civil Engineering & Architecture, Nanyang Normal University, Nanyang 473061, China
First published on 22nd November 2016
Abstract
This paper identifies the performance of irreversible fouling during microfiltration (MF) for surface water treatment. A pilot-scale MF process was conducted and blocking models were employed to fit the fouling behaviors. Results identified intermediate blocking as the major fouling mechanism. Fouled membranes were then chemically cleaned by sodium hypochlorite (oxidant), sodium hydroxide (base) and hydrochloric acid (acid), sequentially. The order of these chemical solutions based on their ability to restore flux was as follows: oxidant > base > acid. Oxidant cleaning greatly decreased the membrane contact angle compared with base/acid cleanings. Hydrophobicity and molecular weight (MW) distribution of the organics in the cleaning solutions were determined by adsorbent resins and high performance size exclusion chromatography (HPSEC) with peak-fitting. Fouled membranes with and without chemical cleaning were analyzed via Fourier transform infrared spectroscopy (FTIR). It was found that low molecular weight acids and humics (LMWAH), humic substances (HS) and biopolymers (BP) in the fractions of both neutral hydrophilic compounds (NEU) and strongly hydrophobic acids (SHA) were responsible for hydraulically irreversible fouling (HIF); this was also associated with humic-like, protein-like and polysaccharide-like substances. LMWAH in the NEU fraction contributed to chemically irreversible fouling (CIF), which was mainly related to polysaccharide-like substances.
1. Introduction
Low pressure membrane (LPM) filtration in surface water treatment has achieved enormous development in the past decades. However, membrane fouling, hydraulically irreversible fouling (HIF) in particular, is still a huge obstacle for membrane technology applications as it decreases productivity, increases energy consumption and reduces membrane life spans.1 Natural organic matters (NOM) are believed to be of great detriment to LPM, and their characteristics such as molecular weight (MW) distribution and hydrophobicity have been the research focus associated with HIF for a long time. Improvements in analytical techniques make it possible to characterize NOM more efficiently. Resin adsorption fractionation and high performance size exclusion chromatography (HPSEC) are the most optimistic measurement methods available for the determination of organic fractions in surface water. With DAX-8/XAD-4/IRA-958 adsorbent resins, NOM could be fractionated into strongly hydrophobic acids (SHA), weakly hydrophobic acids (WHA), charged hydrophilic acids (CHA) and neutral hydrophilic acids (NEU) fractions.2 MW distribution of NOM such as low MW neutrals, low MW acids, building blocks (BB), HS, and BP could also be identified and isolated by HPSEC in conjunction with a relatively new technique named peak-fitting.3–5 Based on advanced analysis technologies, hydrophobic fractions (e.g. humic acid),6 hydrophilic fractions,7 hydrophobic and hydrophilic compounds,8 SHA and NEU fractions,9 low MW compounds,10 and macromolecules BP fractions11 were all once believed to be responsible for irreversible fouling. There were obvious contradictory results about the main foulants in the present research. This may be explained by (1) NOM being complex, various and unstable, thus difficult to control and predict; (2) some results for observing irreversible fouling were obtained based on model water or a bench-scale process, which were lacking in conviction and could not really represent natural water in pilot-/full-scale plant. Therefore, investigation into the characteristics of natural water that causes irreversible fouling in pilot-/full-scale operation is essential to better understand irreversible fouling behaviors.Membrane fouling is usually caused by cake formation12 and pore blocking or restriction.13–15 Hermia proposed four mechanistic models, i.e. complete pore blocking, standard pore blocking, intermediate blocking and cake layer formation, to analyze membrane fouling.16,17 However, an important limitation of Hermia's original models was that it was only applicable for constant-pressure systems. Later, Huang et al. revised Hermia's model and made it suitable for constant-flux filtration.18 The improved models were useful because most full-scale LPM filtration systems were designed and operated in a constant flux. Yet limited literature was reported about the evaluation of the irreversible fouling behavior in a constant-flux operation using the revised blocking model.
Although the membrane was periodically cleaned by air scrubbing or backwashing to reduce fouling, some organic substances still accumulated in/on the membrane during long-term operation, resulting in HIF.11 In general, HIF could be classified as chemically reversible fouling, which is mostly restorable after chemical cleaning, and chemically irreversible fouling (CIF), which cannot be recovered after chemical cleaning and causes long-term permeability loss.19 Much study in previous research has been done to the effect of diverse chemical cleaning reagents on irreversible fouling and to the correlation of cleaning solutions' characteristics with irreversible foulants.20–24 However, these studies were usually focused on HIF, mainly chemically reversible fouling, ignoring CIF – another substantial detriment to the membrane process. A better understanding of the characteristics of CIF is needed for optimization of the chemical cleaning scheme. To date, few studies have been carried out to investigate CIF.
The objectives of the study were (1) to analyze the MF membrane fouling behaviors in constant-flux operation via blocking models; (2) to observe the removal of NOM fractions by the MF membrane process; (3) to identify the components of irreversible foulants via chemical cleaning and (4) to characterize the fouled and cleaned membrane by flux recovery, contact angle and Fourier transform infrared spectroscopy (FTIR). The main novelty of this study is that (1) revised blocking models were used to fit the irreversible fouling behaviors in constant-flux operation; (2) irreversible foulants in the MF process were fractionated into different fractions by adsorbent resins and HPSEC with peak-fitting and (3) chemically irreversible foulants were identified by FTIR in this study. The results were expected to provide useful information for understanding the mechanism of irreversible membrane fouling during surface water treatment.
2. Materials and methods
2.1 Pilot plant and filtrating operation
The pilot experiments were carried out in Chongshan Waterworks, located in Wuxi city. The schematic of its apparatus is illustrated in Fig. S1.† The raw water samples, collected from Taihu Lake, were firstly filtered through 300 μm screens to remove large suspended matters, and then drawn into the raw water tank. Pretreatment was carried out by dosing with polyaluminium chloride, powdered activated carbon and KMnO4 into the reaction tank, allowing mixation and sedimentation by slope plate. A polyvinylidene fluoride (PVDF) hollow fiber MF membrane, supplied by Dow Corporation, was used in this study. As an excellent membrane material, PVDF is widely applied in the membrane industry and drinking water treatment due to its outstanding thermal stability, mechanical strength, and also its distinction in chemical resistance against a wide range of harsh chemicals.25,26 It is valuable and useful to investigate its fouling behavior. The membrane was 0.1 μm in pore size and 20 m2 in filtration area. The submerged membrane module operated in a dead-end, constant-flow filtration mode with a fixed permeation flux of 50 liter per square meter per hour (LMH). The filtration cycle lasted 30 min: 25 min of filtration; 5 min of backwashing by air scrubbing at a flow rate of 1 m3 h−1 and water washing at a flow rate of 2.25 m3 h−1.2.2 Chemical cleaning protocol
At the end of the filtration experiment, the fouled membrane was disassembled for chemical cleaning using three types of chemical agents, i.e. an oxidant (sodium hypochlorite, 0.2 wt%), base (sodium hydroxide, 0.2 wt%) and acid (hydrochloric acid, 0.4 wt%). The fouled membranes were firstly flushed by ultrapure water to remove reversible foulants on the surface, and then put into 1 L cleaning solutions, followed by full stirring and a two-hour soaking. Finally, NOM in the cleaning solutions were isolated into strongly hydrophobic acids (SHA), weakly hydrophobic acids (WHA), charged hydrophilic acids (CHA) and neutral hydrophilic acids (NEU) via adsorbent resins (Supelite DAX-8, Amberlite XAD-4 and Amberlite IRA-958) according to a previous method.272.3 Analytical methods
CODMn was analyzed according to the Chinese State Environmental Protection Agency Standard Methods. UV254 of the water samples were determined by a UV-spectrophotometer (Hach DR5000, USA) and dissolved organic carbon (DOC) by a total organic carbon (TOC) analyzer (Shimadzu TOC-L, Japan). The polysaccharides (PS) and proteins (PN) were measured based on the phenol-sulfuric acid method28 and bicinchoninic acid (BCA) Protein Assay Kit method29 respectively. The MW distribution of DOC was characterized by an HPSEC system (Waters e2695, USA; UV254 detector, Waters 2489, USA; TOC analyzer, Sievers 900 Turbo, USA). The peak-fitting technique was used in chromatogram analysis to settle the overlapping peaks via peak-fitting software (Version 4.12, Systat Software Inc., USA) according to the method explained by Lai et al.5The fouled and cleaned membranes were made into small-sized modules in order to measure water flux recovery. A filtration process, with a setup following the method depicted in previous report,30 was conducted until a constant flow emerged. To confirm the membrane's hydrophilicity, contact angles were measured using a contact angle tensiometer (TL100, Biolin, Sweden).31 The changes of functional groups in fouled and cleaned membranes were detected via an FTIR spectrophotometer (Nicolet 5700, Thermo, USA).
3. Results and discussion
3.1 MF performance and fouling behavior analysis
Fig. S2† presents the treatment efficiency of the hybrid membrane process. It is evident that pretreatment was the main contributor to CODMn and UV254 removal with an average removal efficiency of 64.68% and 64.50%, when MF only increased 9.93% and 1.32% for the removal of CODMn and UV254, respectively. Pretreatment prior to the membrane processes would reduce membrane fouling due to the removal of NOM, resulting in a prolonged membrane lifespan and induced long-term performance. Different pretreatments such as coagulation,32,33 oxidation34–36 and adsorption37–40 before MF have been widely used as methods to mitigate membrane fouling in membranes for drinking water treatment. In this study, hybrid pre-treatments were dosed into the reaction tank. After pretreatment, prominent decrease in CODMn and UV254 were observed, indicating that hybrid pretreatment could be considered as an effective method. The trans-membrane pressure (TMP) changes are depicted in Fig. S3.† As expected, TMP increased with the extending operating time. On the 13th day, TMP reached the limiting value of 0.07 MPa. To profoundly understand the fouling behaviors, four typical filtration models were employed to fit the experimental data,18 and the regression results are exhibited in Fig. 1. The mechanisms of blocking during fouling have been discussed in various studies according to Hermia's four types of blocking mechanisms, i.e. complete pore blocking, intermediate pore blocking, standard pore blocking and cake layer formation.41 Lin et al.42 used bovine serum albumin (BSA) to determine the membrane fouling behavior and found that standard blocking was the main fouling mechanism. Li et al.43 investigated the effects of algae organic matter on the fouling of a poly(ether sulfone) membrane, and the results suggested that cake formation was the major mechanism. As is shown in Fig. 1, the fouling could be well fitted by all four models (R2 > 0.9), yet the blocking models assumed that only one mechanism is active at any given time in the operation cycle, and the best fitting model was regarded as the main fouling mechanism.16 A comparison of R2 values indicated that intermediate blocking was the main fouling mechanism of MF for surface water treatment (R2 = 0.9589), which presumed that each particle reaching the membrane may not only block membrane pores, as in the case of complete pore blocking, but also attach to other particles on the membrane surface.18 An intermediate blocking model has also been proposed by several authors as the main fouling mechanism during the dead-end MF of wastewater.44,45 In other studies, intermediate pore blocking, followed by cake filtration was regarded as the governing mechanism.46–48 This is not surprising because cake layer formation was related to reversible fouling and resulted from the loose attachment of fouling materials to membrane surfaces, which could be removed by physical cleaning methods, e.g. backwash.49 That is to say, cake layer formation will not directly lead to HIF. However, formation of a strong matrix of a fouling layer with solutes during a continuous filtration will result in a reversible fouling being transformed into an irreversible fouling layer, e.g., formation of gel layer under long-term operation.50 This indicated that gel layer formation was directly related to HIF. In addition, pore blocking is also less reversible and is regarded as another type of irreversible fouling.49 Therefore, intermediate blocking, including pore blocking and gel layer formation, was the main mechanism of HIF behavior.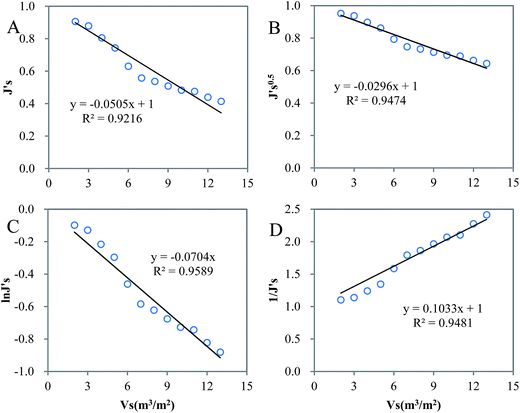 | ||
| Fig. 1 Regression analysis of membrane fouling behavior using models of complete blocking (A), standard blocking (B), intermediate blocking (C) and cake formation (D). | ||
3.2 NOM retained by hybrid membrane process
NOM in raw, pretreated and filtrated water samples were fractionated to SHA, WHA, CHA and NEU fractions by adsorbent resins, and the results are shown in Fig. 2. The fraction contents in raw water samples were in the following order: SHA > NEU > WHA > CHA. After pretreatment, remarkable decrease in SHA and WHA were observed, indicating the effectiveness of pretreatment in removing hydrophobic fractions. Pre-coagulation is a successful treatment for controlling the fouling of MF/UF by the removal of NOM in raw water.39 Commonly, the hydrophobic fraction and high molar mass compounds of NOM are removed more efficiently than the hydrophilic fraction and the low molar mass compounds by coagulation.51–53 The PAC adsorption prior to membrane filtration is also a traditional and reliable pretreatment. As a nonpolar absorbent, PAC exhibited an effective adsorptive capacity for nonpolar matters (i.e. SHA fraction).27 Therefore, a hybrid pretreatment had a better ability of removing hydrophobic fractions compared with hydrophilic fractions.Fig. S4† shows the variations in MW distributions of raw, pretreated and filtered water samples using HPSEC coupled with UV254 and TOC responses. Several peaks were recognized in the raw water sample and the MWs of the resolved peaks were calculated to be 320 Da (Peak 1), 1000 Da (Peak 2), 2500 Da (Peak 3), and 500 kDa (Peak 4), which could probably be associated with the low molecular weight neutrals (LMWN, Peak 1), low molecular weight acids and humics (LMWAH, Peak 2), humic substances (HS, Peak 3), and biopolymers (BP, Peak 4).3–5,27,54 Based on this, MW distributions of the raw water were separated into isolated components by peak-fitting (Fig. 3). Fig. 4 exhibits the content of isolated components in raw, pretreated and filtrated water samples.
 | ||
| Fig. 4 MW components of raw, pretreated and filtrated water samples determined by HPSEC with peak-fitting. | ||
The main component in raw water was the LMWAH fraction, followed by BP and HS, with LMWN only being a small portion. Pretreatment had the ability to remove LMWAH and BP fractions, presumably due to the coagulation and adsorption for macro MWs and small MW removal, respectively.51 BP removal by MF was not surprising because a portion of the BP components was held back by the membrane due to size exclusion.32 Interestingly, a large portion of the LMWAH components was retained after the MF process. In addition, the HS components were also entrapped by the MF membrane. The hydrodynamic sizes of HS and LMWAH, in this study, were estimated to be less than 3.5 nm using an empirical equation.55 Since the mean size of the membrane pores (Φ = 0.1 μm) was much larger than that of HS and LMWAH, membrane pore adsorption, rather than size exclusion, functioned as the main mechanism for these components' removal. It can be obtained from Section 3.1 that intermediate blocking was the main fouling mechanism. Previous reports revealed that small MW components narrowed the membrane pores firstly and high MW components consequently plugged the membrane pores and deposited on the membrane surface.8 Therefore, we believed that not only macro MW components (BP), but also the retained small MW components (LMWA and HS) led to the increase of TMP and HIF.
3.3 Irreversible foulants extracted by chemical cleaning
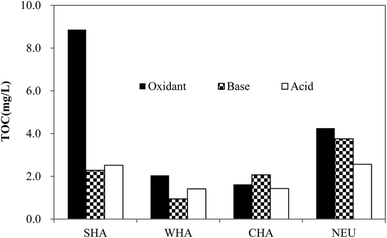
In order to investigate the irreversible fouling, sodium hypochlorite (oxidant), sodium hydroxide (base) and hydrochloric acid (acid) agents were used to extract foulants from the fouled membranes. These cleaning solutions were then fractionated into SHA, WHA, CHA and NEU fractions and the content of each fraction is displayed in Fig. 5. Similar to the composition of raw water, all the three cleaning solutions were dominated by NEU and SHA termed by TOC, whereas WHA and CHA only accounted for a small portion. This result was consistent with the results of Huang's study, i.e. NEU and SHA were the main the compounds in the cleaning solution of alkaline and acidic agents.9 Other studies also claimed that NEU/SHA fractions contributed to membrane fouling greatly.56,57 That is to say, the irreversible foulants of the membrane mainly resulted from the NEU and SHA fractions. This will be further discussed in the following section.Fig. S5† shows the MW distribution of the cleaning solutions. Fig. 6 and 7 exhibit the results of peak-fitting MW of extracted foulants and their corresponding contents.
It can be seen that the foulants in oxidant and base cleaning solutions had a broad range of MW distributions (i.e. from Peak 1 to 4). Sodium hypochlorite showed a better cleaning efficiency than sodium hydroxide in all the peaks. This was due to the fact that oxidizing cleaning oxidized the functional groups to smaller acidic groups, which reduced the adhesion of the foulants to the membranes.49 However, alkaline cleaning could only effectively disintegrate large molecule matters into small molecule matters and the adhesion of the foulants to the membranes remained.58,59 In contrast, acidic cleaning only contained Peaks 1 to 3, failing to remove foulants with MW larger than 10 kD. Acidic cleaning mainly eliminated inorganic fouling that was caused by chemical precipitation of inorganic substances and biologically mineral salts,60,61 and thus was unable to effectively remove organic matters.
NOM with MWs ranging from several hundred to over 100 kDa were reported to have caused severe membrane fouling.62 Fig. 7 reveals that the MWs of organics such as LMWAH and HS were the major foulants, probably because they represented the NOM fractions with lower MWs, and could be adsorbed to the internal pore walls and lead to irreversible membrane fouling. Some researchers previously supported our discovery and believed that small organic molecules could bring about serious membrane fouling.56,57,63 In addition to the two small-MW matters, Peak 4, representing BP, also had an evident TOC response. BP could pollute the membranes more readily through forming a gel layer on the membrane surface. This was in agreement with the results of prior researchers. For instance, L. Fan et al. concluded that macromolecule organics (>30 kDa) mainly caused rapid flux decline;57 Huck et al.,64 Zheng et al.65 and Henderson et al.66 also found that the BP fractions were the main retained organic fractions after membrane treatment. In order to further clarify the effect of MW distribution and hydrophobicity on MF performance, HPSEC combined with adsorbent resins were used to determine component percentage of MWs in SHA and NEU fractions (Fig. S6†). SHA and NEU, which mainly contained LMWAH and HS components as well as a small portion of the BP fraction, were primarily responsible for the HIF. The SHA fraction contained numerous aliphatic, aromatic compounds and unsaturated carbon structures,2 which could be retained by membranes due to the electrostatic repulsions between the membrane and the foulants.67 Similar results were obtained by Nilson et al. who found that humic acid was the major foulant.68 The NEU fraction, composed of macro MW substances BP such as PS, PN and colloidal organics,69 was also responsible for the membrane fouling. Fig. S7† testifies that PS and PN in the cleaning solutions were mainly from NEU fractions, irrespective of the type of cleaning solution. The other three fractions (SHA, WHA and CHA) contained less PS and PN. A comparison of PS and PN in the three cleaning solutions showed that oxidizing cleaning had a stronger ability to elute both PS and PN compared with alkaline cleaning and acidic cleaning. The NEU fraction also contained LMW substances such as aliphatic alcohols, poly functional alcohols, short-chain aliphatic amines, amides, aldehydes, ketones, esters and cyclic amides.70 Therefore, it was presumable that these LMWAH in both SHA and NEU fractions could block and narrow the membrane pores.
3.4 Flux recovery, contact angle and FTIR of membrane
Fig. 8 depicts the degree of flux recovery after chemical cleaning of the fouled membranes. Compared with the flux for the fouled membrane, the water fluxes after sodium hypochlorite, sodium hydroxide and hydrochloric acid cleanings were respectively 3.71, 2.00 and 1.25 times. The oxidizing agent showed the best cleaning effect among the tested solutions, and this was consistent with previous reports. Espinasse et al.71 investigated the chemical cleaning efficiencies by different cleaning reagents (i.e. deionized water, HCl, NaOH, NaClO and Ultrasil) and found that NaClO appeared to have removed all traces of deposits, both organic and inorganic, followed by NaOH. Zhang et al.72 used NaOH, NaClO, HCl and EDTA to remove foulants from a UF membrane and the results indicated that NaClO exhibited the best performance (88.4% ± 1.1%) in removing the irreversible fouling resistance. Fig. 9 shows the contact angles of the virgin, fouled and cleaned membranes: the virgin membrane showed an original contact angle of 96.4°, indicating a slightly hydrophobic nature; after foulants' accumulation, the contact angle of the membrane increased to 101.7°, suggesting the deposition of hydrophobic foulants on the surface; after chemical cleaning, the contact angle of the cleaned membrane was decreased to a certain extent, implying the removal of more hydrophobic organic foulants and the lowering of the membrane hydrophobicity as a consequence. Oxidizing cleaning brought about a greater decrease in the membrane contact angle than compared with base/acid; this was consistent with previous research.71 The slight hydrophilicity after oxidizing cleaning may be attributable to the fact that either foulants or cleaning chemicals or both had modified the membrane surface. As mentioned above, the oxidant was able to increase the hydrophilicity of the parent constituents.49 In addition, researchers reported that sodium hypochlorite modified the physical–chemical properties of the membranes by changing the contact angle of the membrane.73 Levitsky et al.74 also found a decrease in the contact angle of the membrane after soaking in NaClO. Those modifications would be due to the elimination of the residues of the preservatives. Another possible cause was that some hydrophilic inorganic particles still adhered to the membrane surface even after cleaning, and thus were responsible for the decrease in the contact angle. | ||
| Fig. 8 Effect of chemical cleaning on recovery of flux of fouled membrane (J0: pure water flux before chemical cleaning, J: pure water flux after chemical cleaning). | ||
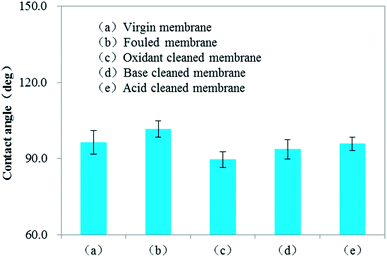 | ||
| Fig. 9 Contact angle of the (a) virgin membrane, (b) fouled membrane, (c) oxidant cleaned membrane, (d) base cleaned membrane and (e) acid cleaned membrane. | ||
As shown in Fig. 10, FTIR spectra were obtained to analyze the composition of the foulants on the virgin, fouled and cleaned membrane surfaces. The virgin membrane showed the basic wavelengths of a PVDF material at 840 cm−1, 875 cm−1, 975 cm−1, 1082 cm−1, 1180 cm−1 and 1400 cm−1, which corresponded to CH2 rocking, C–C asymmetric stretching, PVDF fingerprint, CF2 symmetric stretching, CF out of plane deformation and CH2 wagging, respectively.75,76 When the membrane was fouled, transmittance intensity of some of the peaks (875 cm−1 and 975 cm−1) decreased, probably caused by the obstruction of some membrane pores by foulants. An evident absorptive band appeared in the high wave region, i.e. 2900–3600 cm−1, and the peak was concentrated at about 3400 cm−1 (Fig. 10), which was related to OH stretching vibrations (acidic groups). In the FTIR spectra, it was reported that the characteristic peaks at 1515–1650 cm−1 and 3300 cm−1 corresponded to the peptide bond in protein-like substances, and peaks of polysaccharide-like substances could be found at 1000–1260, 2925 and 3200–3550 cm−1.77 In our research, the band near 1400 cm−1, which was associated with the O–H bend in COOH group, broadened and this could be ascribed to the fouling caused by humic substances.78 The peak at 1650 cm−1 corresponded to functional groups of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N and N–H and belonged to amide I,79,80 indicating the existence of protein-like substances. The appearance of another broad band at 1040 cm−1 was attributable to the C–O of carbohydrates, indicating the existence of polysaccharide-like substances during the membrane fouling.81 Based on the abovementioned analysis of the FTIR spectra, it can be confirmed that humic-like, protein-like and polysaccharide-like substances were the main hydraulically irreversible foulants. According to the reports of previous literatures, NEU contained a high portion of polysaccharide-like and protein-like compounds encapsulated within HS aggregates,4 and the breakdown products of macro MW polysaccharide-like matters corresponded to amorphous carbon sugars, alcohols, ketones carbohydrates and amino sugars.2 SHA was associated with the fulvic-acid-like substances and humic-acid-like substances,82 low molecular weight acids and nitrogen containing aromatics, conjugated unsaturated acids, etc.3,83 That is to say, the foulants, i.e. humic-like, protein-like and polysaccharide-like substances determined by FTIR were related to these NEU and SHA fractions (based on fractionation, Fig. 5), and LMWAH, HS and BP (based on HPSEC, Fig. 7).
C, C–N and N–H and belonged to amide I,79,80 indicating the existence of protein-like substances. The appearance of another broad band at 1040 cm−1 was attributable to the C–O of carbohydrates, indicating the existence of polysaccharide-like substances during the membrane fouling.81 Based on the abovementioned analysis of the FTIR spectra, it can be confirmed that humic-like, protein-like and polysaccharide-like substances were the main hydraulically irreversible foulants. According to the reports of previous literatures, NEU contained a high portion of polysaccharide-like and protein-like compounds encapsulated within HS aggregates,4 and the breakdown products of macro MW polysaccharide-like matters corresponded to amorphous carbon sugars, alcohols, ketones carbohydrates and amino sugars.2 SHA was associated with the fulvic-acid-like substances and humic-acid-like substances,82 low molecular weight acids and nitrogen containing aromatics, conjugated unsaturated acids, etc.3,83 That is to say, the foulants, i.e. humic-like, protein-like and polysaccharide-like substances determined by FTIR were related to these NEU and SHA fractions (based on fractionation, Fig. 5), and LMWAH, HS and BP (based on HPSEC, Fig. 7).
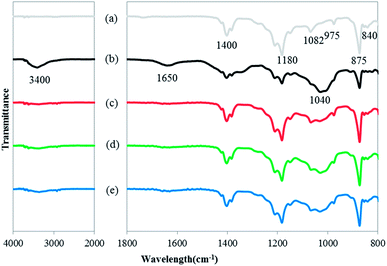 | ||
| Fig. 10 FTIR spectra of the (a) virgin membrane, (b) fouled membrane, (c) oxidant cleaned membrane, (d) base cleaned membrane and (e) acid cleaned membrane. | ||
After being cleaned by the three different chemical agents sequentially, all the peaks (3400 cm−1, 1650 cm−1 and 1040 cm−1) for foulants weakened or even disappeared, confirming the effectiveness of chemical cleaning in eliminating membrane foulants. Among the three chemical cleaning reagents, NaClO exhibited the best performance for the removal of these foulants, which was supported by previous research that revealed the ability of NaClO to eliminate the major foulants such as carbohydrate-like and protein-like materials.72 Nevertheless, the bands of polysaccharide-like substances remained, suggesting the failure of chemical cleaning to remove these components, resulting in the CIF. The possible reason was that protein-like substances mainly deposited onto the membrane surface and could be easily removed by chemical cleaning, whereas polysaccharide-like substances were mainly adsorbed on the membrane pore walls and could hardly be extracted by chemical cleaning.84 These results suggested that polysaccharide-like substances had a greater potential, compared with protein-like substances, to cause CIF. As mentioned in Section 3.3, polysaccharide-like substances (i.e. PS) were mainly included in NEU fractions (Fig. S7†), whereas the other three fractions (SHA, WHA and CHA) contained relatively less PS. It is also testified that pore blocking was caused by LMWAH. Thus it is not difficult to speculate that LMWAH in NEU, mostly the breakdown products of macro MW polysaccharide-like matters,2 was mainly responsible for CIF.
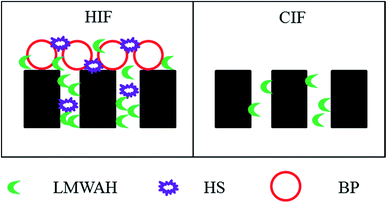
Based on the abovementioned results, the HIF and CIF during MF of surface water is illustrated in Fig. 11.
It can be observed from Fig. 11 that during the MF process of surface water, HIF was caused by (1) pore blocking, i.e. adsorption of LMWAH and HS into the pores, and (2) formation of a gel layer, i.e. deposition of BP in conjunction with LMWAH and HS on the membrane surface. Pore blockage occurs due to the full or partial closure of membrane pores by particles.85 It usually happens rapidly in the initial stages of filtration when the membrane surface is bare of deposit, and the incoming particles can have a direct interaction with a membrane pore. Gel formation is caused by the consolidation of a layer of highly concentrated macromolecules in the immediate vicinity of the membrane surface.23 These components, i.e. LMWAH, HS and BP were mainly from NEU and SHA fractions, which were also associated with humic-like, protein-like and polysaccharide-like substances. HS and BP could be removed by chemical cleaning, yet only LMWAH remained in the pores after chemical cleaning, which contributed significantly to the CIF. This component was mainly from NEU fractions that were related to polysaccharide-like substances.
In this study, lake water was used for samples. In order to make the conclusion more useful, the characteristics of the water used in this study were compared with water from other sources, and similar water characteristics, i.e. hydrophobicity and molecular weight distribution, were found in other water samples. Fig. S8† shows the fractions of different water sources fractionated by adsorbent resins. Clearly, SHA and NEU were the major fractions in all the four water samples. Fig. S9† exhibits the molecular weight distribution of water samples from different sources, determined by HPSEC with peak-fitting. A comparison of the components in different water samples revealed that the LMWAH fraction was the main component, followed by HS and BP, whereas LMWN only accounted for a small fraction in all of the four waters. Therefore, the conclusions obtained in this study may be applicable to more surface water samples. To better elucidate the water characteristics on membrane fouling, investigation on the MF process of distinct natural water is necessary in the future.
4. Conclusion
Based on the analysis of irreversible fouling on the MF process for surface water treatment, the following conclusions may be drawn. Intermediate blocking was the major fouling mechanism. LMWAH, HS and BP in both NEU and SHA fractions were responsible for HIF, which were also associated with humic-like, protein-like and polysaccharide-like substances; LMWAHs in the NEU fraction contributed greatly to the CIF, which was mainly related to polysaccharide-like substances.Acknowledgements
This research was financially supported by the National Water Pollution Control and Treatment Key Technologies R&D Program (No. 2012ZX07403-001).References
- S. Peldszus, C. Hallé, R. H. Peiris, M. Hamouda, X. Jin, R. L. Legge, H. Budman, C. Moresoli and P. M. Huck, Water Res., 2011, 45, 5161–5170 CrossRef CAS PubMed.
- S. Wong, J. V. Hanna, S. King, T. J. Carroll, R. J. Eldridge, D. R. Dixon, B. A. Bolto, S. Hesse, G. Abbt-Braun and F. H. Frimmel, Environ. Sci. Technol., 2002, 36, 3497–3503 CrossRef CAS PubMed.
- C. W. Chow, R. Fabris, J. V. Leeuwen, D. Wang and M. Drikas, Environ. Sci. Technol., 2008, 42, 6683–6689 CrossRef CAS PubMed.
- L. Xing, R. Fabris, C. W. Chow, J. van Leeuwen, M. Drikas and D. Wang, J. Environ. Sci., 2012, 24, 1174–1180 CrossRef CAS.
- C.-H. Lai, Y.-C. Chou and H.-H. Yeh, J. Membr. Sci., 2015, 474, 207–214 CrossRef CAS.
- D. Jermann, W. Pronk, S. Meylan and M. Boller, Water Res., 2007, 41, 1713–1722 CrossRef CAS PubMed.
- K. Kimura, H. Yamamura and Y. Watanabe, Sep. Sci. Technol., 2006, 41, 1331–1344 CrossRef CAS.
- H. Yamamura, K. Kimura and Y. Watanabe, Environ. Sci. Technol., 2007, 41, 6789–6794 CrossRef CAS PubMed.
- W. Huang, H. Chu, B. Dong, M. Hu and Y. Yu, Desalination, 2015, 355, 99–109 CrossRef CAS.
- C. Ayache, M. Pidou, J. Croué, J. Labanowski, Y. Poussade, A. Tazi-Pain, J. Keller and W. Gernjak, Water Res., 2013, 47, 2633–2642 CrossRef CAS PubMed.
- H. Yamamura, K. Okimoto, K. Kimura and Y. Watanabe, Water Res., 2014, 54, 123–136 CrossRef CAS PubMed.
- S. Ognier, C. Wisniewski and A. Grasmick, Membr. Technol., 2002, 2002, 6–10 CrossRef.
- J. Lee, W.-Y. Ahn and C.-H. Lee, Water Res., 2001, 35, 2435–2445 CrossRef CAS PubMed.
- H. Choi, K. Zhang, D. D. Dionysiou, D. B. Oerther and G. A. Sorial, Sep. Purif. Technol., 2005, 45, 68–78 CrossRef CAS.
- R. Liu, X. Huang, L. Chen, X. Wen and Y. Qian, Process Biochem., 2005, 40, 125–130 CrossRef CAS.
- J. Hermia, Trans. Inst. Chem. Eng., 1982, 60, 183–187 CAS.
- A. Grenier, M. Meireles, P. Aimar and P. Carvin, Chem. Eng. Res. Des., 2008, 86, 1281–1293 CrossRef CAS.
- H. Huang, T. A. Young and J. G. Jacangelo, Environ. Sci. Technol., 2007, 42, 714–720 CrossRef.
- M. Zupančič, D. Novak, J. Diaci and I. Golobič, Desalination, 2014, 344, 321–328 CrossRef.
- J. Gu, H. Zhang, Z. Zhong and W. Xing, Ind. Eng. Chem. Res., 2011, 50, 11245–11251 CrossRef CAS.
- Y. Mo, J. Chen, W. Xue and X. Huang, Sep. Purif. Technol., 2010, 75, 407–414 CrossRef CAS.
- Z. Wang, Q. Zhang, Y. Ma, L. Yang, Y. Lyu and S. Zhao, Desalination, 2013, 325, 122–131 CrossRef CAS.
- X. Shi, G. Tal, N. P. Hankins and V. Gitis, Journal of Water Process Engineering, 2014, 1, 121–138 CrossRef.
- C. Regula, E. Carretier, Y. Wyart, G. Gésan-Guiziou, A. Vincent, D. Boudot and P. Moulin, Water Res., 2014, 56, 325–365 CrossRef CAS PubMed.
- N. A. Hashim, L. Fu and K. Li, J. Membr. Sci., 2009, 345, 134–141 CrossRef CAS.
- M. L. Yeow, Y. T. Liu and K. Li, J. Appl. Polym. Sci., 2003, 90, 2150–2155 CrossRef CAS.
- J. Liu, H. Chu, B. Dong, J. Wang, Y. Sheng and H. He, Desalin. Water Treat., 2014, 52, 6878–6885 CrossRef CAS.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. T. Rebers and F. Smith, Anal. Chem., 1956, 28, 350–356 CrossRef CAS.
- P. Smith, R. I. Krohn, G. Hermanson, A. Mallia, F. Gartner, M. Provenzano, E. Fujimoto, N. Goeke, B. Olson and D. Klenk, Anal. Biochem., 1985, 150, 76–85 CrossRef CAS PubMed.
- W. Huang, H. Chu, B. Dong and J. Liu, Desalination, 2014, 344, 329–338 CrossRef CAS.
- H. Celebi and A. Kurt, Carbohydr. Polym., 2015, 133, 284–293 CrossRef CAS PubMed.
- R. Peiris, M. Jaklewicz, H. Budman, R. Legge and C. Moresoli, Water Res., 2013, 47, 3364–3374 CrossRef CAS PubMed.
- K. Kimura, K. Tanaka and Y. Watanabe, Water Res., 2014, 49, 434–443 CrossRef CAS PubMed.
- J. P. Chen, S. Kim and Y. Ting, J. Membr. Sci., 2003, 219, 27–45 CrossRef CAS.
- W. Yu, N. J. Graham and G. D. Fowler, Water Res., 2016, 95, 1–10 CrossRef CAS PubMed.
- W. Yu, L. Xu, N. Graham and J. Qu, Sci. Rep., 2014, 4, 6513 CrossRef CAS PubMed.
- J. Haberkamp, A. S. Ruhl, M. Ernst and M. Jekel, Water Res., 2007, 41, 3794–3802 CrossRef CAS PubMed.
- Y. Matsui, H. Hasegawa, K. Ohno, T. Matsushita, S. Mima, Y. Kawase and T. Aizawa, Water Res., 2009, 43, 5160–5170 CrossRef CAS PubMed.
- H. Huang, K. Schwab and J. G. Jacangelo, Environ. Sci. Technol., 2009, 43, 3011–3019 CrossRef CAS PubMed.
- X. J. Gai and H. S. Kim, Desalination, 2008, 225, 288–300 CrossRef CAS.
- M. Said, A. Ahmad, A. W. Mohammad, M. T. M. Nor and S. R. S. Abdullah, J. Ind. Eng. Chem., 2015, 21, 182–188 CrossRef CAS.
- S. H. Lin, C. L. Hung and R. S. Juang, Chem. Eng. J., 2008, 145, 211–217 CrossRef CAS.
- L. Li, Z. Wang, L. C. Rietveld, N. Gao, J. Hu, D. Yin and S. Yu, Environ. Sci. Technol., 2014, 48, 14549–14557 CrossRef CAS PubMed.
- J. A. Suarez and J. M. Veza, Desalination, 2000, 127, 47–58 CrossRef CAS.
- I. Rosas, S. Collado, A. Gutiérrez and M. Díaz, J. Membr. Sci., 2014, 465, 27–33 CrossRef CAS.
- C. Duclos-Orsello, W. Li and C. C. Ho, J. Membr. Sci., 2006, 280, 856–866 CrossRef CAS.
- T. C. Arnot, R. W. Field and A. B. Koltuniewicz, J. Membr. Sci., 2000, 169, 1–15 CrossRef CAS.
- S. Mondal and S. De, Sep. Purif. Technol., 2010, 75, 222–228 CrossRef CAS.
- Z. Wang, J. Ma, C. Y. Tang, K. Kimura, Q. Wang and X. Han, J. Membr. Sci., 2014, 468, 276–307 CrossRef CAS.
- Z. Wang, Z. Wu, X. Yin and L. Tian, J. Membr. Sci., 2008, 325, 238–244 CrossRef CAS.
- W. Sun, J. Liu, H. Chu and B. Dong, Membranes, 2013, 3, 226–241 CrossRef CAS PubMed.
- M. Sillanpää and A. Matilainen, Chapter 3–NOM Removal by Coagulation, 2015 Search PubMed.
- L. Zheng, C. Huaqiang, D. Bingzhi, S. Fei and L. Hao, Desalin. Water Treat., 2012, 30, 80–88 CrossRef.
- N. H. Lee, G. Amy, J. P. Croue and H. Buisson, Water Res., 2004, 38, 4511–4523 CrossRef CAS PubMed.
- P. Aimar, M. Meireles and V. Sanchez, J. Membr. Sci., 1990, 54, 321–338 CrossRef CAS.
- T. Carroll, S. King, S. R. Gray, B. A. Bolto and N. A. Booker, Water Res., 2000, 34, 2861–2868 CrossRef CAS.
- L. H. Fan, J. L. Harris, F. A. Roddick and N. A. Booker, Water Res., 2001, 35, 4455–4463 CrossRef CAS PubMed.
- H. Yu, Z. Wang, Q. Wang, Z. Wu and J. Ma, Chem. Eng. J., 2013, 231, 206–213 CrossRef CAS.
- C. Liu, S. Caothien, J. Hayes, T. Caothuy, T. Otoyo and T. Ogawa, Membrane chemical cleaning: From art to science, Pall Corporation, Port Washington, NY, 2001, p. 11050 Search PubMed.
- F. Meng, S.-R. Chae, A. Drews, M. Kraume, H.-S. Shin and F. Yang, Water Res., 2009, 43, 1489–1512 CrossRef CAS PubMed.
- L. Malaeb, P. Le-Clech, J. S. Vrouwenvelder, G. M. Ayoub and P. E. Saikaly, Water Res., 2013, 47, 5447–5463 CrossRef CAS PubMed.
- A. W. Zularisam, A. F. Ismail and R. Salim, Desalination, 2006, 194, 211–231 CrossRef CAS.
- C. W. Li and Y. S. Chen, Desalination, 2004, 170, 59–67 CrossRef CAS.
- P. M. Huck, S. Peldszus, J. Haberkamp and M. Jekel, Environ. Sci. Technol., 2009, 43, 3878–3884 CrossRef.
- X. Zheng, M. Ernst and M. Jekel, Water Res., 2009, 43, 238–244 CrossRef CAS PubMed.
- R. K. Henderson, N. Subhi, A. Antony, S. J. Khan, K. R. Murphy, G. L. Leslie, V. Chen, R. M. Stuetz and P. Le-Clech, J. Membr. Sci., 2011, 382, 50–59 CrossRef CAS.
- C.-F. Lin, S.-H. Liu and O. J. Hao, Water Res., 2001, 35, 2395–2402 CrossRef CAS PubMed.
- J. Nilson and F. Digiano, Journal, 1997, 89, 4 Search PubMed.
- E. Filloux, J. Labanowski and J.-P. Croue, Bioresour. Technol., 2012, 118, 460–468 CrossRef CAS PubMed.
- W. Buchanan, F. Roddick, N. Porter and M. Drikas, Environ. Sci. Technol., 2005, 39, 4647–4654 CrossRef CAS PubMed.
- B. P. Espinasse, S.-R. Chae, C. Marconnet, C. Coulombel, C. Mizutani, M. Djafer, V. Heim and M. R. Wiesner, Desalination, 2012, 296, 1–6 CrossRef CAS.
- Y. Zhang, J. Tian, H. Liang, J. Nan, Z. Chen and G. Li, J. Environ. Sci., 2011, 23, 529–536 CrossRef CAS.
- P. Wang, Z. Wang, Z. Wu, Q. Zhou and D. Yang, Chem. Eng. J., 2010, 162, 1050–1056 CrossRef CAS.
- I. Levitsky, A. Duek, R. Naim, E. Arkhangelsky and V. Gitis, Chem. Eng. Sci., 2012, 69, 679–683 CrossRef CAS.
- Y. C. Woo, J. J. Lee, L. D. Tijing, H. K. Shon, M. Yao and H.-S. Kim, Desalination, 2015, 369, 51–61 CrossRef CAS.
- V. Puspitasari, A. Granville, P. Le-Clech and V. Chen, Sep. Purif. Technol., 2010, 72, 301–308 CrossRef CAS.
- F. Zhao, Y. Su, X. Tan, H. Chu, Y. Zhang, L. Yang and X. Zhou, Colloids Surf., B, 2015, 136, 431–439 CrossRef CAS PubMed.
- F. J. Rodríguez, P. Schlenger and M. García-Valverde, Sci. Total Environ., 2016, 541, 623–637 CrossRef PubMed.
- M. Luján-Facundo, J. Mendoza-Roca, B. Cuartas-Uribe and S. Álvarez-Blanco, J. Food Eng., 2015, 163, 1–8 CrossRef.
- I. Levitsky, A. Duek, E. Arkhangelsky, D. Pinchev, T. Kadoshian, H. Shetrit, R. Naim and V. Gitis, J. Membr. Sci., 2011, 377, 206–213 CrossRef CAS.
- K. Kimura, Y. Hane, Y. Watanabe, G. Amy and N. Ohkuma, Water Res., 2004, 38, 3431–3441 CrossRef CAS PubMed.
- H. Q. Chu, Y. L. Zhang, X. F. Zhou and B. Z. Dong, J. Membr. Sci., 2013, 433, 126–134 CrossRef CAS.
- B. P. Allpike, A. Heitz, C. A. Joll, R. I. Kagi, G. Abbt-Braun, F. H. Frimmel, T. Brinkmann, N. Her and G. Amy, Environ. Sci. Technol., 2005, 39, 2334–2342 CrossRef CAS PubMed.
- K.-J. Hwang and Y.-C. Chiang, Sep. Purif. Technol., 2014, 125, 74–82 CrossRef CAS.
- R. W. Field and J. J. Wu, Desalination, 2011, 283, 68–74 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra18284d |
| This journal is © The Royal Society of Chemistry 2016 |