DOI:
10.1039/C6RA18254B
(Paper)
RSC Adv., 2016,
6, 92502-92509
Fabrication of reduced graphene oxide–bimetallic Pd@Au nanocomposites for simultaneous determination of ascorbic acid, dopamine and uric acid†
Received
18th July 2016
, Accepted 11th September 2016
First published on 13th September 2016
Abstract
In this work, we have successfully developed a facile method for the fabrication of Pd@Au core–shell (Pd@Au) heterostructures using Pd nanocubes as the structure-directing cores. The glassy carbon electrode (GCE) modified by the Pd–Au core–shell (Pd@Au)/RGO was fabricated and employed to simultaneously determine concentrations of ascorbic acid (AA), dopamine (DA) and uric acid (UA). Because of the synergistic effect among Au, Pd and RGO, the Pd@Au/RGO/GCE demonstrates excellent electrocatalytic activity, electron transfer capability, selectivity and sensitivity in detection of AA, DA and UA. TEM images and EDX mapping showed that the as-synthesized Pd@Au nanoparticles were in Pd core and Au shell nanostructures, distributed homogeneously on the reduced graphene oxide. Cyclic voltammetries (CVs) and differential pulse voltammetry (DPV) were used to evaluate the electrochemical behaviors of AA, UA and DA on the as-fabricated electrode. Three sharp and separate oxidation peaks were observed with the co-existence of AA, DA and UA. Good linear calibration plots for AA, DA and UA were established by simultaneously increasing the concentration of AA, DA and AA in the ranges of 50–2856.63 μM, 1–400.56 μM and 5–680.76 μM, respectively. The individual DPVs for AA, DA and UA were also investigated and detection limits estimated were 24.88 μM, 0.2 μM and 1.25 μM, respectively. Moreover, the modified electrode demonstrated high performance when it was applied to test the content of AA, DA and UA in the analysis of real samples.
1. Introduction
Ascorbic acid (AA) is an indispensable nutrient in humans and some animals, while AA cannot be self-produced by humans. AA works as an antioxidant and protects humans from threats of free radicals; AA is also a type of coenzyme. The human body can get it from fresh vegetables and fruits.1,2 AA deficiency could lead to scurvy. Dopamine (DA), synthesized in the brain and kidneys, is an important neurotransmitter; it is largely responsible for feelings such as pleasure, joy and excitement. DA is also linked to addiction. According to recent research, DA plays an important role in the treatment of depression and Alzheimer's disease. DA deficiency leads to loss of muscle control or Parkinson's disease. Restoring DA concentrations helps in controlling or eliminating diverse disease conditions.3–6
Uric acid (UA), the main metabolite for birds and reptiles, is the primary end metabolic product for purine. For healthy humans, a small amount of UA can be excreted from the human body through biological fluids such as urine, feces and sweat. Abnormal content of UA in blood may lead to some diseases such as gout, arthritis, gouty nephropathy, hyperuricemia and anemia.7,8 To diagnose, track and treat these diseases, detecting these molecules with satisfactory accuracy, low detection limits and high sensitivity is critically important. Due to the electrochemical activity of AA, DA and UA, electrochemical techniques have attracted great attention because of high sensitivity, ease of handling and fabrication, cost effectiveness and short response time.8–11 However, only one broad oxidation peak is observed on bare GCE since DA, AA and UA have very close oxidation potentials. It's necessary to prepare an electrode with higher selectivity and sensitivity for simultaneous detection DA, AA and UA.12–14
Recently there have been a lot of studies on graphene–metal nanocomposites acting as electrochemical biosensors.15,16 Graphene, as an allotrope of carbon in the form of a 2D one-atom-thick sp2-bonded carbon sheet, honey-comb lattice, has many extraordinary properties such as excellent electrical conductivity (550 S cm−1), locally conjugated structure, large specific surface area (2630 m2 g−1), large amount of edge-plane-like defects and high chemical, thermal and electrochemical stability.17–20 These characteristics make graphene an excellent candidate for applications in determination of DA, UA and AA.21–23
Nanomaterials have been playing a more and more important role in manufacturing, environmental protection and new energy resources due to their special properties; nanotechnology has various applications in catalysis, biomedicine, fine chemicals and sensors, among others.24–28 The electrochemical activity for synthesized sensors may be affected not only by the morphology of the particles including size and shape but also elemental composition. Due to synergistic and special effects among components, multicomponent nanostructures such as bimetallic hierarchical heterogeneous structures have drawn great attention. The core–shell bimetallic nanoparticles, which is a good approach to couple the catalytic performances of one metal with another metal, have been studied intensively.29,30 Compared with other bimetallic nanoparticles, Au@Pd nanoparticles have proven to have more efficient catalytic properties in various reactions.31,32 Compared to Au@Pd nanoparticles, Au shell on Pd@Au nanoparticles has less catalytic activity than Pd shell on Au@Pd nanoparticles and is relatively less studied. Because Au shell is biocompatible and stable, it is a good choice for fabrication of sensors; properties of Au shell can be effectively adjusted by the Pd core.
Based on advantages of graphene and Au@Pd nanoparticles, development of Pd@Au/RGO electrochemical sensors is promising. The synergetic effects of the following three factors make the fabricated Pd@Au/RGO nanocomposite a good sensor: (1) ability of RGO to facilitate dispersion of Pd@Au nanoparticles on RGO sheets, which leads to an increased reactive surface area; (2) ability of RGO to retard aggregation Pd@Au nanoparticles and enhance stability of the Pd@Au/RGO nanocomposite; and (3) ability of RGO to adsorb detected molecules and thus facilitate easy detection of the same.33–37
In this work, Au-decorated Pd heterogeneous nanocomposite was first synthesized, followed by addition of reduced graphene oxide (RGO). The combination of RGO and Pd@Au nanoparticles can increase electrode surface and enhance optical, electronic, and catalytic properties. The RGO modified Pd@Au nanocomposite was assembled onto the surface of GCE, resulting in large surface area and high electron transfer ability. The RGO sheets and Pd@Au nanoparticle-modified GCE (RGO/Pd@Au/GCE) was employed to simultaneously determine the AA, UA and DA. For comparison, the electrocatalytic performance of RGO-modified glassy carbon electrode (RGO/GCE), and Pd@Au-modified glassy carbon electrode (Pd@Au/GCE) towards AA, UA and DA oxidation were also compared.
2. Experimental section
2.1 Reagents and materials
Graphite powder, palladium chloride powder (PdCl2, 99%), hydrogen tetrachloroaurate tetrahydrate (HAuCl4·4H2O, 99.9%), cetyltrimethylammonium bromide (CTAB, 99%), C2H5OH, HCl, KOH, potassium ferrocyanide, potassium ferricyanide, disodium hydrogen phosphate, and sodium dihydrogen phosphate were obtained from Sinopharm Chemicals Reagent Co., Ltd. (China) and used as received. Ascorbic acid (AA), uric acid (UA) and dopamine (DA) were purchased from Acros Organics and used without further purification. All chemicals were of analytical reagent grade. Mouse urine and serum were obtained from Soochow University Medical School. Secondary distilled water was used throughout the experiment. Preparation of graphene oxide (GO) nanosheets was based on the literature.38,39
2.2 Apparatus
All electrochemical experiments were executed with a CHI660 B electrochemical workstation (Shanghai Chenhua Instrument Plant, China) with a three-electrode system. A conventional glassy carbon electrode (GCE, 3 mm diameter) fabricated via the as-synthesized nanocomposite, a saturated calomel electrode (SCE) and a platinum wire were considered as the working electrode, reference electrode and counter electrode, respectively. Also 0.1 M phosphate buffer solution (PBS) (pH = 7.0) was used as an electrolyte solution. All experiments were conducted at room temperature. The as-prepared nanomaterials were characterized by TEM on a TECNAI-G20 electron microscope with an accelerating voltage of 200 kV to determine morphologies. EDS was utilized to investigate element distributions via elemental mappings on a JEM-2100F electron microscope. Structural information for the nanocomposite was obtained via XRD (X'Pert-Pro MPD, PANalytical Company).
2.3 Synthesis of Pd–Au core–shell (Pd@Au) nanoparticles
The H2PdCl4 (22.56 mM) aqueous solution was first obtained by thoroughly dissolving 0.2 g PdCl2 powder in 25 mL of 90.2 mM HCl aqueous solution and diluting to 50 mL with secondary distilled water in a 50 mL volumetric flask. 100 mg CTAB were dissolved in 30 mL of secondary distilled water at 95 °C in a round-bottom flask, followed by rapid addition of 200 μL of 22.56 mM H2PdCl4 under vigorous stirring. Two minutes later, 3.0 mL of freshly prepared AA solution (0.03 M) were slowly added to the above mixture. The reaction continued for 30 min, and then 930 μL HAuCl4 (24.3 mM) aqueous solution and 3 mL AA (0.01 M) aqueous solution were added. The reaction was maintained for 1 h more under continuous stirring at 95 °C and then terminated by cooling down to room temperature. The final solution was centrifuged and washed with secondary distilled water several times to remove impurities. The obtained product was dispersed into 10 mL secondary distilled water by sonication to form Pd@Au nanoparticles for further use.
2.4 Preparation of 1 g L−1 GO solution
Graphene oxide (GO) nanosheets were prepared as reported.36,37 1 mg mL−1 GO nanosheets solution was prepared by dispersing 10 mg of obtained GO powder into 10 mL of secondary distilled water under sonification for 90 min and then impurities were centrifuged.
2.5 Synthesis of reduced graphene oxide modified Pd@Au nanocomposite
1 mL obtained GO solution (1 g L−1) was added into a round-bottom flask and diluted to 10 mL, followed by introducing 1 mL hydrazine hydrate. The solution was stirred for 2 h to obtain the RGO solution. Then 3 mL above prepared Pd@Au nanoparticle solution was added and the mixture mixed for 1 h under stirring to form the Pd@Au/RGO nanocomposite solution.
2.6 Electrode preparation
The GCE (3 mm in diameter) was carefully polished using 0.05 μm alumina slurries on a wet polishing cloth to obtain a mirror-like surface. The electrode was then washed ultrasonically first in ethanol and then in water for several minutes, sequentially. Finally, it was rinsed thoroughly with secondary distilled water and dried in air. 10 μL of the obtained Pd@Au/RGO, RGO, and Pd@Au solutions were drop cast onto clean GCE and then dried under air to obtain the Pd@Au/RGO/GCE, RGO/GCE, and Pd@Au/GCE, respectively. The values of electrochemical active surface area (ECSA) of RGO/GCE, Pd@Au/GCE, and Pd@Au/RGO/GCE were calculated based on the Randles–Sevcik equation, assuming mass transport only by the diffusion process.40,41 5.0 mM K3Fe(CN)6/K4Fe(CN)6 containing 1.0 M KCl was prepared for this detection:
| Ip = 2.69 × 105AD1/2n3/2γ1/2C, |
where n is the number of electrons participating in the reaction, A is the electroactive surface area, D is the diffusion coefficient of the molecule (6.707 ± 0.02 × 10−6 cm2 s−1), C is the analyte concentration (mol cm−3), and γ is the scan rate (V s−1). The calculated ECSA for GCE, RGO/GCE, Pd@Au/GCE, and Pd@Au/RGO/GCE was 0.0833 cm2, 0.1036 cm2, 0.108 cm2, 0.191 cm2, respectively.
3. Results and discussion
3.1 Characterization of Pd@Au/RGO nanocomposite
Pd@Au bimetallic nanoparticles hybridized with RGO nanosheets could be obtained easily using a simple method in this work. The different magnification morphologies of prepared nanostructures including Pd, Pd@Au and Pd@Au/RGO were characterized by transmission electron microscopy (TEM), as shown in Fig. 1–3. High TEM magnifications (Fig. 1(C) and (D) and Fig. 2(C) and (D)) show that the synthesized Pd and Pd@Au nanoparticles are in nanocubes and nearly octahedral structured shapes, respectively. Low-magnification TEM images (Fig. 3(A) and (B)) show that the Pd@Au are uniformly distributed on the wrinkled RGO surface. The average size of the synthesised Pd, Pd@Au nanoparticles is estimated to be 28 nm and 70 nm, respectively. Fig. 4 shows the EDX analysis including elements mapping on Pd and Au as well as the line scan result of the obtained Pd@Au nanocomposite. EDX mapping on Au and Pd reveals that the synthesized Au-decorated Pd bimetallic nanoparticles are of Pd core Au shell structure. The Pd is covered and wrapped by the Au element. The Au is grown on the Pd seed and the structure is mediated and undercontrolled. The shape of Pd@Au nanoparticle changes to a near octahedron structure from the Pd cubic nanostructure.
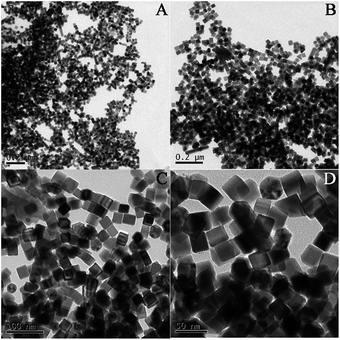 |
| | Fig. 1 Typical TEM images of Pd cubes with different magnifications, A and B are low magnification, C and D are high magnification. | |
 |
| | Fig. 2 Typical TEM images of Pd@Au core–shell with different magnifications, A and B are low magnification, C and D are high magnification. | |
 |
| | Fig. 3 Typical TEM images of Pd@Au/RGO nanocomposite in different magnifications, A and B are low magnification, C and D are high magnification. | |
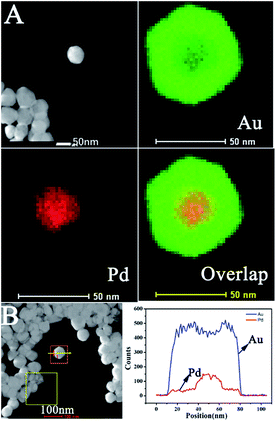 |
| | Fig. 4 (A) EDX mapping for Au, Pd and overlap. (B) Line scan for elements of Au and Pd. | |
Structures of the synthesized RGO, Pd@Au and Pd@Au/RGO were further analyzed by XRD, as presented in Fig. S1(A).† Both the Pd@Au and Pd@Au/RGO nanocomposites show five diffraction peaks at 38.3°, 44.6°, 64.7°, 77.5° and 82.4°, which are attributed to (111), (200), (220), (311), and (222) reflections of face-centered cubic (fcc) structure. No obvious typical diffraction peaks of Pd are detected in the as-prepared Pd@Au and Pd@Au/RGO composites, indicating the Pd is totally covered by Au, which is consistent with EDX mapping results. As expected, the diffraction signal from (111) is the dominant and strongest peak due to its preferred orientation while depositing on the substrates. The broad peak around 24.31° in the synthesized Pd@Au/RGO can be attributed to the (002) facet of the crystalline graphite, which indicates that the RGO was reduced to RGO.42
3.2 Cyclic voltammetric behaviors of AA, DA and UA on the modified electrodes
Fig. S1(B)† shows the cyclic voltammetry responses (CVs) of the four electrodes in 5.0 mM Fe(CN)63−/4− and 0.1 M KCl solution at a scan rate of 50 mV s−1. The Pd@Au/RGO/GCE electrode exhibits a remarkable enhancement in the redox peak current (147.4 μA) compared to the peak currents of 84.35 μA, 80.66 μA and 64.77 μA for Pd@Au/GCE, RGO/GCE and GCE, respectively, implying that the Pd@Au/RGO assembled sensor has the best electrocatalytic activity. Meanwhile, much narrower peak potential difference is observed for Pd@Au/RGO/GCE, which also indicates a much higher efficiency in electron transfer at the interface of the electrode Pd@Au/RGO/GCE compared with the other three. This is attributed to the synergistic effect among Au, Pd and RGO. The addition of Pd@Au to RGO can improve the electrochemical activity of the obtained electrode likely due to the high surface area of the Pd@Au/RGO/GCE. The higher ECSA value of Pd@Au/RGO/GCE was likely contributed to possession of more active sites, which significantly enhanced its electrocatalytic activity. Moreover, the excellent conductivity of RGO contributes to transfer electrons between the redox probe and electrode surface.
3.3 Cyclic voltammetric detection of AA, DA and UA
Fig. 5(A)–(C) shows the CV curves of AA, DA and UA on bare GCE, RGO/GCE, Pd@Au/GCE and Pd@AU/RGO/GCE, respectively. Shown in Fig. 5(A), the anodic oxidation peak currents for the 5.0 mM AA solution in 0.1 M PBS (pH = 7) are 65.53 μA, 37.35 μA and 43.92 μA and no obvious oxidation peak for Pd@Au/RGO/GCE, RGO/GCE, Pd@Au/GCE and bare GCE, respectively. Fig. 5(B) shows the anodic oxidation peak currents for the 0.1 mM DA solution in 0.1 M PBS (pH = 7) at 57.51 μA, 41.66 μA, 17.71 μA and 13.58 μA, respectively, for Pd@Au/RGO/GCE, RGO/GCE, Pd@Au/GCE and bare GCE. Finally, Fig. 5(C) shows that the anodic oxidation peak currents for the 0.5 mM UA solution in 0.1 M PBS (pH = 7) are 69.92 μA, 37.67 μA, 37.13 μA and 12.50 μA, respectively. From above investigations, we can conclude that the Pd@Au/RGO/GCE demonstrates the best electrocatalytic performance toward electrooxidation of AA, DA and UA compared with the RGO/GCE and Pd@Au/GCE electrodes. Factors such as unique characteristics, super electron conductivity, larger amount of edge plane-like defects for RGO and high surface to volume ratio of noble metals (Pd and Au), the synergistic interaction effect between Pd and Au, the special nanostructure of Pd and Au in the core–shell structure, and the extremely excellent capability of Au in electrochemical detection and so on have enabled the as-prepared Pd@Au/RGO/GCE for great electrochemical activity in the determination of AA, DA and UA with the highest electron transfer ability.
 |
| | Fig. 5 CVs of bare GCE, RGO/GCE, Pd@Au/GCE, and Pd@Au/RGO/GC recorded in 0.1 M PBS (pH = 7), including 5.0 mM AA (A), 0.1 mM DA (B) and 0.5 mM UA (C) PBS (pH = 7.0) at scan rate of 50 mV s−1. | |
3.4 Electrochemical parameters of DA, AA and AA at Pd@Au/RGO modified electrode
The scan rate impact on the electrochemical responses of Pd@Au/RGO was studied. Fig. S2(A)–(C)† presents the CVs of 5.0 mM AA, 0.1 mM DA and 0.5 mM UA in 0.1 M PBS (pH = 7) at different scan rates from 20 to 200 mV s−1, respectively. According to previously discussed investigations, we can see that all of the anodic and cathodic peak potentials for DA, AA and UA gradually shift positively and negatively, respectively, with the increase of scan rate in the range of 20 to 200 mV s−1, which indicates that there is no adsorption of DA, AA and UA on Pd@Au/RGO in the 0.1 M PBS solution.43
The anodic oxidation peak currents increased with scan rate increment, and the currents are linearly regressed with the scan rate ranging from 20 to 200 mV s−1. Regression equations for AA, DA and UA follow:
| Ipa (μA) = 0.223v (mV s−1) + 29.362 (AA), R2 = 0.997; |
| Ipa (μA) = 0.5727v (mV s−1) + 2.7287 (UA), R2 = 0.994; |
and
| Ipa (μA) = 1.0584v (mV s−1) + 0.2707 (UA), R2 = 0.997. |
The correlation coefficients are 0.997, 0.994 and 0.997 for AA, DA and UA, respectively. These results suggest that all oxidations toward AA, DA and UA on Pd@Au/RGO are adsorption-controlled processes.13 Moreover, due to redox peak potentials varying with the scan rate, conclusions can be made that the electrochemical reactions of AA, DA and UA are quasi-reversible.44
The influences of solution acidity on the electrochemical oxidation peak currents and peak potentials of AA, DA and UA were also investigated by running CVs in different PBS pH from 5.0 to 9.0. As shown in Fig. S3(A)–(C),† as pH value of the solution increases, oxidation peak potentials for AA, DA and UA decrease gradually, which proves that the protons are involved in the electrochemical reaction.45
The anodic peak potentials are observed to be in linear relationships with the pH value. Regression equations for AA, DA and UA follow:
| Upa (mV) = −52pH + 482 (AA), R2 = 0.963; |
| Upa (mV) = −59pH + 697 (DA), R2 = 0.928; |
and
| Upa (mV) = −63pH + 839 (UA), R2 = 0.995. |
The slopes of AA, DA and UA are −52 mV/pH, −59 mV/pH, and −63 mV/pH, respectively. These obtained slopes are fairly close to the theoretical value as −59 mV/pH at 25 °C, which is consistent with the Nernest equation,46 suggesting that the electron transfer in the electrochemical reactions is equal to the number of protons. As a result, two electrons and two protons take part in the electrochemical oxidation reaction of AA, DA and UA.
3.5 Simultaneous detection of AA, DA and UA in modified electrode
Measurements of the simultaneous determination of AA, DA and UA were performed on the synthesized Pd@Au/RGO/GCE, Pd@Au/GCE, and RGO/GCE. Fig. 6 shows the CVs in co-existing 5.0 mM AA, 0.1 mM DA and 0.5 mM UA in 0.1 M PBS (pH = 7) for the Pd@Au/RGO/GCE, Pd@Au/GCE, and RGO/GCE. For Pd@Au/RGO/GCE, the better separated oxidation peaks are observed at the peak potentials of 0.15 V, 0.28 V and 0.41 V with corresponding peak currents of 100.9 μA, 128.7 μA, and 123.5 μA. When RGO/GCE is applied as the biosensor, there are only two separate anodic peaks detected at peak potentials of 0.30 V and 0.45 V with corresponding peak currents of 89.16 μA and 75.89 μA. However, only one peak is observed on both Pd@Au/GCE and bare GCE, with the anodic peak currents of 90.72 μA and 62.67 μA, respectively. It's clearly observed that the as-prepared Pd@Au/GCE demonstrates the best electrocatalytic capability toward the electrochemical detection of AA, DA and UA. Only Pd@/Au/RGO/GCE is capable of determining AA, DA and UA simultaneously. The differences for the three anodic peak potentials are big enough to simultaneously detect AA, DA and UA when Pd@Au/RGO is used to fabricate the GCE. Mutual interaction effects among Pd, Au and RGO enable synthesized Pd@Au/RGO/GCE the best electron transfer capability and highest electrochemical activity, which makes possible simultaneous detection of AA, DA and UA. Performance of the proposed biosensor was compared with that of AA, DA and UA sensors based on other matrices as shown in Table 1.
 |
| | Fig. 6 CVs of bare GCE, RGO/GCE, Pd@Au/GCE, and Pd@Au/RGO/GCE in 0.1 M PBS (pH = 7.0) solution containing 5.0 mM AA, 0.1 mM DA, 0.5 mM UA at scan rate of 50 mV s−1. | |
Table 1 Comparison of proposed DA, UA and AA biosensors with other biosensors based on selected electrodes
| Electrodes |
Detection limit (μM L−1) |
Linearity range (μM L−1) |
Reference |
| AA |
DA |
UA |
AA |
DA |
UA |
| SGNF/IL/CS/GCE |
14.8 |
0.04 |
0.1 |
30–350 |
0.05–240 |
0.12–260 |
47 |
| HNP-PtTi |
24.2 |
3.2 |
5.3 |
200–1000 |
4–500 |
100–1000 |
48 |
| Pd/CNF-CPE |
15.0 |
0.2 |
0.7 |
50–4000 |
2.0–200 |
0.5–160 |
49 |
| PtNPs-MWCNT/GCE |
20 |
0.05 |
0.35 |
24.5–765 |
0.06–2.03 |
0.46–50 |
50 |
| Pd@Au/RGO/GCE |
24.88 |
0.2 |
1.25 |
50–2856.63 |
1–400.56 |
5–680.76 |
This work |
Differential pulse voltammetry (DPV) has a wide application in electrochemistry detection due to its higher sensitivity, better signal-to-noise and lower detection limit. In this work, DPV was used to monitor both the individual and simultaneous determination of AA, DA and UA. The electrochemical process for AA, DA and UA was tested via different concentrations of AA, DA and UA. Shown in Fig. 7(A) are the DPV curves of various concentrations of AA, DA and UA. The anodic peak currents for AA, DA and UA were observed to be in linear relationships with the corresponding concentrations of the three molecules in the concentration ranges of 50–2856.63 μM, 1–400.56 μM and 5–680.76 μM for AA, DA and UA, respectively. Fig. 7(B)–(D) depicts the corresponding linear regression plots between the oxidation peak currents versus concentrations of AA, DA and UA, the regression equations as follows:
| Ipa (μA) = 0.0266C (μM) + 48.39 (AA) (R2 = 0.997), |
| Ipa (μA) = 0.3001 (μM) + 57.00 (DA) (R2 = 0.998) |
and
| Ipa (μA) = 0.1730C (μM) + 21.73 (UA) (R2 = 0.995). |
 |
| | Fig. 7 DPV curves of Pd@Au/RGO/GCE in 0.1 M PBS (pH = 7.0) solution containing different concentrations of AA from 50 to 2856.63 μM, DA from 1 to 400.56 μM and UA from 5 to 680.76 μM (A). (B)–(D) Plots of anodic currents vs. concentration of AA, DA and UA based on DPV curves (A), respectively. | |
Detection limits for AA, DA and UA are 50 μM, 1 μM and 5 μM, respectively. To further evaluate the electrocatalytic performance of the as-synthesized Pd@Au/RGO/GCE electrode, the electrochemical reaction was also tested by separately increasing the concentration of AA, DA and UA in the 0.1 M PBS solution (pH = 7.0). Fig. S4(A)–(C)† shows individual DPV curves for the anodic peak currents with different concentrations of AA, DA and UA. The figure inset depicts the corresponding linear relationships between the oxidation peak currents and concentration of AA, DA and UA, respectively:
| Ipa (μA) = 0.0149C (μM) + 15.40 (AA) (R2 = 0.951), |
| Ipa (μA) = 16.257C (μM) + 4.803 (DA) (R2 = 0.953) |
and
| Ipa (μA) = 1.15299C (μM) + 10.62 (UA) (R2 = 0.965). |
Detection limits for the AA, DA and UA are 24.88 μM, 0.2 μM and 1.25 μM, respectively, which are much lower than detection limits for simultaneous detection of AA, DA and UA. All above results demonstrate that the prepared Pd@Au/RGO/GCE can be applied to the simultaneous determination of AA, DA and UA.
3.6 Reproducibility, stability and real sample analysis
Six Pd@Au/RGO/GCE electrodes prepared in the same procedure were employed to test the response of the aqueous solution containing 5.0 mM AA, 0.1 mM DA, and 0.5 mM UA in 0.1 M PBS (pH = 7) to evaluate reproducibility. Results show that the relative standard deviations (RSDs) were 3.2%, 1.5% and 2.0% for AA, DA and UA, respectively, demonstrating good reproducibility. Stability for the as-synthesized Pd@Au/RGO/GCE was also tested by storing the modified electrode at room temperature and CVs were measured every 10 days for 2 months under the same conditions. The relative standard deviations for the oxidation peak currents for AA, DA and UA were 4.5%, 2.8% and 3.6%, respectively, showing good stability.
The prepared Pd@Au/RGO/GCE electrode was applied to measure AA, UA and DA in mouse urine and serum samples to investigate the practicability of the electrode for routine analysis. All samples were diluted by 0.1 M PBS (pH = 7) and analyzed via electrochemical methods. Recovery for AA, DA and UA was done using the standard adding method. Recovery for AA, DA and UA was found to be 95.1%, 98.7% and 96.1%, respectively. The good recovery shows that the prepared Pd@Au/RGO/GCE can be used in simultaneous determinations of AA, UA and DA in real samples.
4. Conclusions
In conclusion, a simple and easy method was developed to synthesize the Pd@Au/RGO nanocomposite. The glassy carbon electrode (GCE) modified by Pd–Au core–shell (Pd@Au)/RGO was fabricated and employed to simultaneously determine concentrations of ascorbic acid (AA), dopamine (DA) and uric acid (UA). Because of the synergistic effect among Au, Pd and RGO, the Pd@Au/RGO/GCE demonstrates excellent electrocatalytic activity, electron transfer capability, selectivity and sensitivity towards the detection of AA, DA and UA. In comparison with the other electrodes such as Pd@Au/GCE and RGO/GCE, the Pd@Au/RGO/GCE demonstrates the best electrocatalytic performance, highest electron transfer capability, and best selectivity and sensitivity towards the detection of AA, DA and UA. Moreover, excellent performance in detecting AA, DA and UA in the real sample observed when using the obtained electrode of Pd@Au/RGO/GCE illustrates the high feasibility in real-life application of the electrode. This work could provide incentive for further utilization of the reduced graphene oxide-modified bimetallic nanocomposite in detecting electroactive small molecules.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 51373111), State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Notes and references
- O. Arrigoni and M. C. C. Tullio, Biochim. Biophys. Acta, 2002, 1569, 1 CrossRef CAS.
- J. F. Ping, J. Wu, Y. X. Wang and Y. B. Ying, Biosens. Bioelectron., 2012, 34, 70 CrossRef CAS PubMed.
- A. Salimi, K. Abdi and G. R. Khayatian, Microchim. Acta, 2004, 144, 161 CrossRef CAS.
- A. Ciszewski and G. Milczarek, Anal. Chem., 1999, 71, 1055 CrossRef CAS PubMed.
- Y. Zhao, Y. Gao, D. Zhan, H. Liu, Q. Zhao, Y. Kou, Y. Shao, M. Li, Q. Zhuang and Z. Zhu, Talanta, 2005, 66, 51 CrossRef CAS PubMed.
- M. Mallesha, R. Manjunatha, C. Nethravathi, G. S. Suresh, M. Rajamathi, J. S. Melo and T. V. Venkatesha, Bioelectrochemistry, 2011, 81, 104 CrossRef CAS PubMed.
- J. Du, R. R. Yue, Z. Q. Yao, F. X. Jiang, Y. K. Du, P. Yang and C. Y. Wang, Colloids Surf., A, 2013, 419, 94 CrossRef CAS.
- A. A. Ensafi, M. Taei, T. Khayamian and A. Arabzadeh, Sens. Actuators, B, 2010, 147, 213 CrossRef CAS.
- F. X. Jiang, R. R. Yue, Y. Du, J. Xu and P. Yang, Biosens. Bioelectron., 2013, 44, 127 CrossRef CAS PubMed.
- C. Q. Wang, J. Du, H. W. Wang, C. E. Zou, F. X. Jiang, P. Yang and Y. K. Du, Sens. Actuators, B, 2014, 204, 302 CrossRef CAS.
- L. Wu, L. Feng, J. Ren and X. Qu, Biosens. Bioelectron., 2012, 34, 57 CrossRef CAS PubMed.
- L. Wang, X. Qin, S. Liu, Y. Luo, A. Asiri, A. Al-Youbi and X. Sun, ChemPlusChem, 2012, 77, 19 CrossRef CAS.
- J. Du, R. R. Yue, F. F. Ren, Z. Q. Yao, F. X. Jiang, P. Yang and Y. K. Du, Biosens. Bioelectron., 2014, 53, 220 CrossRef CAS PubMed.
- B. B. Yang, J. Wang, D. Bin, M. S. Zhu, P. Yang and Y. K. Du, J. Mater. Chem. B, 2015, 3, 7440 RSC.
- Z. H. Sheng, X. Q. Zheng, J. Y. Xu, W. J. Bao, F. B. Wang and X. H. Xia, Biosens. Bioelectron., 2012, 34, 125 CrossRef CAS PubMed.
- C. L. Sun, H. H. Lee, J. M. Yang and C. C. Wu, Biosens. Bioelectron., 2011, 26, 3450 CrossRef CAS PubMed.
- S. C. Sultan and U. Anik, Talanta, 2014, 129, 523 CrossRef CAS PubMed.
- Y. Tepeli and U. Anik, Electrochem. Commun., 2015, 57, 31 CrossRef CAS.
- D. B. Altuntas, Y. Tepeli and U. Anik, 2D Mater., 2016, 3, 034001 CrossRef.
- Y. Tepeli and U. Anik, Electroanalysis, 2016, 28, 1 CrossRef.
- T. Q. Xu, Q. L. Zhang, J. N. Zheng, Z. Y. Lv, J. Wei, A. J. Wang and J. J. Feng, Electrochim. Acta, 2014, 115, 109 CrossRef CAS.
- C. L. Sun, H. H. Lee, J. M. Yang and C. C. Wu, Biosens. Bioelectron., 2011, 26, 3450 CrossRef CAS PubMed.
- D. Chen, L. H. Tang and J. H. Li, Chem. Soc. Rev., 2010, 39, 3157 RSC.
- C. C. Huang, C. Li and G. Q. Shi, Energy Environ. Sci., 2012, 5, 8848 CAS.
- K. H. Ghanbari and N. Hajheidari, Anal. Biochem., 2015, 473, 53 CrossRef CAS PubMed.
- J. Soleymani, Trends Anal. Chem., 2015, 72, 27 CrossRef CAS.
- W. Cheng and R. G. Compton, Trends Anal. Chem., 2014, 58, 79 CrossRef CAS.
- Y. J. Kang and C. B. Murray, J. Am. Chem. Soc., 2010, 132, 7568 CrossRef CAS PubMed.
- A. Wang and Q. Peng, Chem. Mater., 2011, 23, 3217 CrossRef CAS.
- H. L. Wu, C. H. Chen and M. H. Huang, Chem. Mater., 2009, 21, 110 CrossRef CAS.
- T. V. Choudhary, C. Sivadinarayana, A. K. Datye, D. Kumar and D. W. Goodman, Catal. Lett., 2003, 86, 1 CrossRef CAS.
- J. K. Edwards, B. Solsona, P. Landon, A. F. Carley, A. Herzing, M. Watanabe, C. J. Kiely and G. J. Hutchings, J. Mater. Chem., 2005, 15, 4595 RSC.
- J. Li, C. Fan, F. Xiao, R. Yan, S. Fan, F. Zhao and B. Zeng, Electrochim. Acta, 2007, 52, 6178 CrossRef CAS.
- J. Lu, S. Liu, S. Ge, M. Yan, J. Yu and X. Hu, Biosens. Bioelectron., 2012, 33, 29 CrossRef CAS PubMed.
- F. Cui and X. Zhang, J. Electroanal. Chem., 2012, 669, 35 CrossRef CAS.
- K. Shanmugasundaram, P. Subramanian, M. Paramasivam, G. A. Iyengar and K. P. Lee, Gold Bull., 2011, 44, 37 CrossRef CAS.
- Y. Yue, G. Hu, M. Zheng, Y. Guo, J. Cao and S. Shao, Carbon, 2012, 50, 107 CrossRef CAS.
- Z. Q. Yao, M. S. Zhu, F. X. Jiang, Y. K. Du, C. Y. Wang and P. Yang, J. Mater. Chem., 2012, 22, 13707 RSC.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- C. Wang, R. Yuan, Y. Q. Chai, Y. Zhang, F. X. Hu and M. H. Zhang, Biosens. Bioelectron., 2011, 30, 315 CrossRef CAS PubMed.
- L. Wang and Y. Yamauchi, Chem. Mater., 2009, 21, 3562 CrossRef CAS.
- C. Zhai, M. Zhu, Y. Lu, F. Ren, C. Wang, Y. Du and P. Yang, Phys. Chem. Chem. Phys., 2014, 16, 14800 RSC.
- J. Du, R. R. Yue, F. F. Ren, Z. Q. Yao, F. X. Jiang, P. Yang and Y. K. Du, Gold Bull., 2013, 46, 137 CrossRef.
- S. F. Hou, M. L. Kasner, S. J. Su, K. Patel and R. Cuellari, J. Phys. Chem. C, 2010, 114, 14915 CAS.
- G. Liu, H. Chen, G. Lin, P. Ye, X. Wang, Y. Jiao, X. Guo, Y. Wen and H. Yang, Biosens. Bioelectron., 2014, 56, 26 CrossRef CAS PubMed.
- C. Lim, H. Hoh, P. Ang and K. Loh, Anal. Chem., 2010, 82, 7387 CrossRef CAS PubMed.
- X. Niu, W. Yang, H. Guo, J. Ren, F. Yang and J. Gao, Talanta, 2012, 99, 984 CrossRef CAS PubMed.
- D. Zhao, G. Yu, K. Tian and C. Xu, Biosens. Bioelectron., 2016, 82, 119 CrossRef CAS PubMed.
- J. S. Huang, Y. Liu, H. Q. Hou and T. Y. You, Biosens. Bioelectron., 2008, 24, 632 CrossRef CAS PubMed.
- Z. Dursun and B. Gelmez, Electroanalysis, 2010, 22, 1106 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra18254b |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 






